| Steps |
Project |
Process |
Cusabio Features |
Lead Time |
| 1 |
Plasmid construction |
Codon optimization; gene synthesis |
Multiple vectors optimization, More options for customers
Optimize multiple vectors at the same time; Select the vector that has the highest yield, which can shorten lead time; |
5-8 business days |
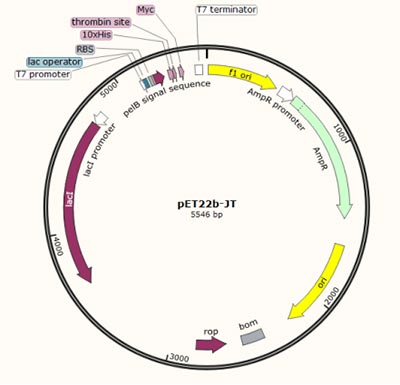
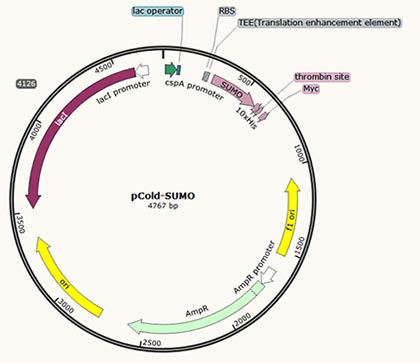
| Restriction digestion of PCR products; Ligation to expression vector, e.g. Pcold-SUMO, pGEX-4T-1, pET22b-JT, etc. |
| Transform TOP10 E.coil competent cells |
| Obtain the correct recombinant plasmid |
| 2 |
Transformation and strain screening |
Transform the recombinant plasmid to host cells, e.g. BL21 (DE3), Rosetta-gami B (DE3) pLysS, C41 cells, culture overnight at 37℃ |
Multi-conditions optimization, multi-hosts selection
In the small test, the temperature and IPTG are optimized to obtain the most suitable culture conditions. Multiple hosts are transformed at the same time to select the host bacteria with the highest yield. |
2-3 business days |
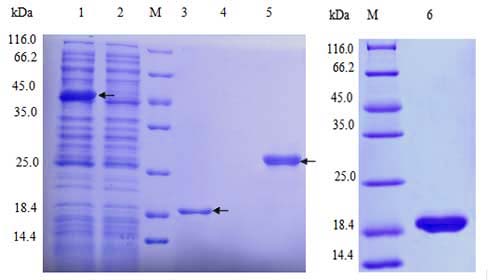
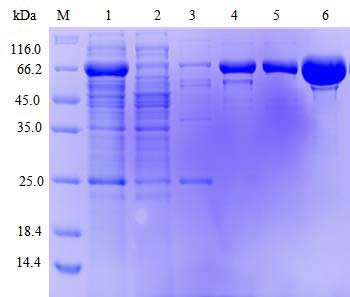
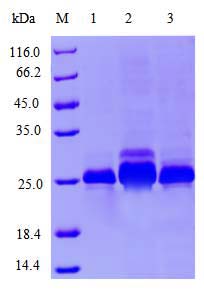
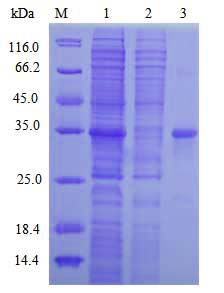
| Select single colony for small-scale induced expression; Detect protein expression by SDS-PAGE; Preserve the best colony. |
| Optimize the expression conditions |
| 3 |
Target protein expression and purification |
1-10 L large-scale expression |
Multiple purification methods (optional)
Explore different chromatographic conditions including ion exchange, hydrophobic and others by using AKTA, and then determine the optimal purification method.
|
5-9 business days |
| Protein purification |
| 4 |
Additional services (Optional) |
Charge |
Tag removal by restriction digestion |
Flexible additional services
Customers can flexibly choose from a variety of additional services to their specific needs, e.g. Endotoxin removal, Filter-sterilization, Tag removal, Lyophilization, etc. Some are complimentary, and some require additional charge.
|
3 business days |
| Free |
Filter-sterilization; Endotoxin removal; Lyophilization (Note: Lyophilization and Filter-sterilization can not be met simultaneously)
|
2 business days |
| 5 |
Quality Control |
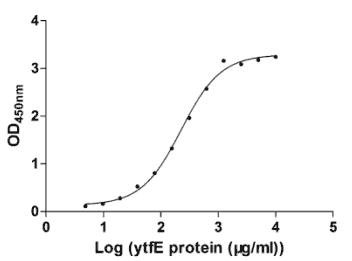
Testing of purity, concentration, etc. QC report is provided. |
Detailed COA report
Detailed product data sheet and COA are provided for each project.
|
3-5 business days |
| Total lead time |
15-25 business days |