| Steps |
Project |
Process |
Cusabio Features |
Lead Time |
| 1 |
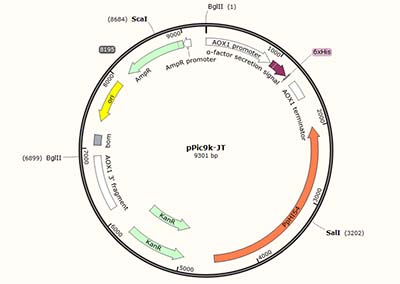
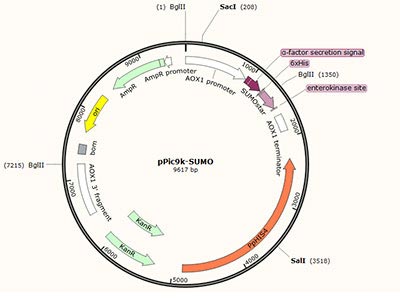
Expression vector construction |
Codon optimization; gene synthesis |
Multiple vectors optimization, More options for customers
In order to improve the success rate of expression and achieve higher yield, in addition to conventional N-terminal fusion protein expression, we also provide protein with C-terminal fusion label, in greater degree to ensure the activity while ensuring the purity. |
8-10 business days |
| The PCR amplification products are ligated to the expression vectors e.g. pPic9k, pPic3.5k, pPiczαA, etc. |
| Transform ligation mixtures into E. coli strain |
| Obtain the correct recombinant plasmid |
| 2 |
Transformation and strain identification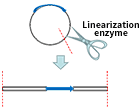 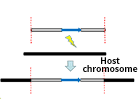 |
Prepare the recombinant plasmid in large quantities |
High copy screening
Conduct multiple screening through unique screening markers of different vectors, and the highest expression level strain was gradually obtained. |
PickRight Technology
The high expression level strain was obtained directly after transformation, and the time was shorten by 5-10 business days compared with the traditional screening. (Theoretically this technology is mainly recommended for the production of less than 5 mg/L protein expression) |
5-8 business days |
| Linearization of recombinant plasmid |
| Transform to GS115, X33, KM71 and other hosts by electroporation |
| PCR analysis is recommended to verify successful transformants |
| Use geneticin G418, Zeocion and other antibiotics for multiple copies screening to obtain high copy |
| 3 |
Small test, scale up expression and purification |
Small scale expression screening (20-40 strains) |
9-12 business days/11-17 business days (Featured Purification)
|
| Determine strain and optimize expression conditions |
|
| Scale up culture |
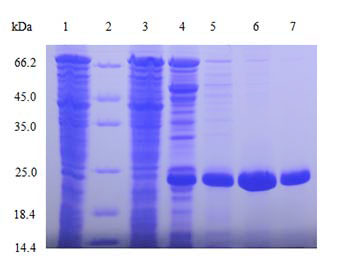
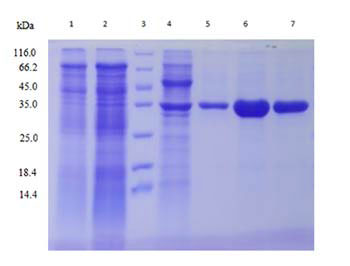
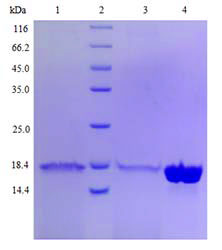
| Protein purification |
Multiple purification methods (optional)
Explore different chromatographic conditions including ion exchange, hydrophobic and others by using AKTA, and then determine the optimal purification method. |
| 4 |
Additional services (Optional) |
Charge |
Tag removal service |
Flexible additional services
Customers can flexibly choose from a variety of additional services to their specific needs, e.g. Endotoxin removal, Filter-sterilization, Tag removal, Lyophilization, etc. Some are complimentary, and some require additional charge.
|
3 business days |
| Free |
Filter-sterilization; Endotoxin removal; Lyophilization
(Note: Lyophilization and filter-sterilization can not be met simultaneously)
|
2 business days |
| 5 |
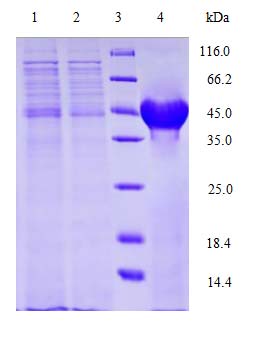
Quality Control |
Testing of purity, concentration, etc. QC report is provided. |
Detailed COA Report
Detailed product data sheet and COA are provided for each project.
|
3-5 business days |
| Total lead time |
25-35 business days |