Recombinant Antibodies for Drug Discovery
Antibodies and fusion proteins are milestones in the history of drug development and have been the main types of biologics. The administration of biological drugs to patients carries a risk of eliciting anti-drug antibodies (ADAs) [1] [2]. This risk is higher if the biotherapeutic is a 'non-self' substance, e.g., a protein replacement therapy to treat monogenic diseases such as severe hemophilia A or B, Pompe's disease [3].
Monitoring the potential Immune response (IR) of biological (e.g., protein) drugs has become a regulatory requirement in drug development and post-marketing. The commonly used tests include: the detection of binding drug antibodies, anti-drug neutralizing antibody detection, etc. It is necessary to prepare antibodies for ADAs detection in clinical samples, and the antibodies can also be used in pharmacokinetics/ pharmacodynamics (PK/PD) studies of protein drugs. Almost all biological drugs cause an anti-drug antibody (ADA) response, or immune response. In the process of pharmacokinetic research, the evaluation of anti-drug resistance antibody (ADA) has become a prominent issue. ADA may have a significant impact on PK research results, and the preparation of anti-drug antibody for ADAs detection is indispensable.
CUSABIO is committed to assisting you in drug development and has developed a series of recombinant antibodies for drugs discovery. These recombinant antibodies are expressed in mammalian cell and have been validated in activity against proteins. So all these antibodies can be used as positive controls in drug discovery or used to verify the activity of the corresponding proteins.
If you don't find any antibody you desired, learn more about CUSABIO Lead Antibody Molecule Discovery Service.
Partial Case Validation Display
● Antibody specificity validation by FC
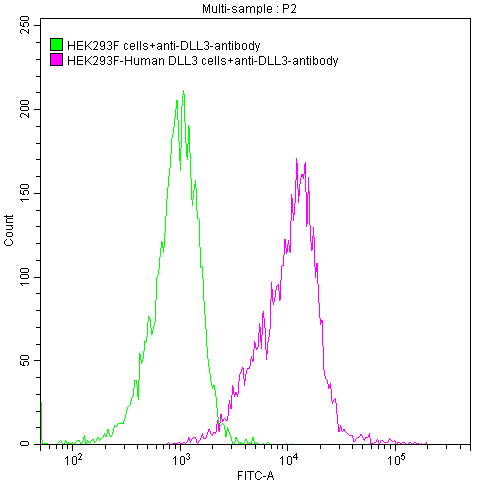
DLL3 Monoclonal Antibody (CSB-RA882142A1HU)
Untransfected HEK293 cells (green line) and transfected Human DLL3 HEK293 stable cells (red line) were stained with anti-DLL3 antibody (2µg/1*106 cells), washed and then followed by FITC-conjugated anti-Human IgG1 Fc antibody and analyzed with flow cytometry.
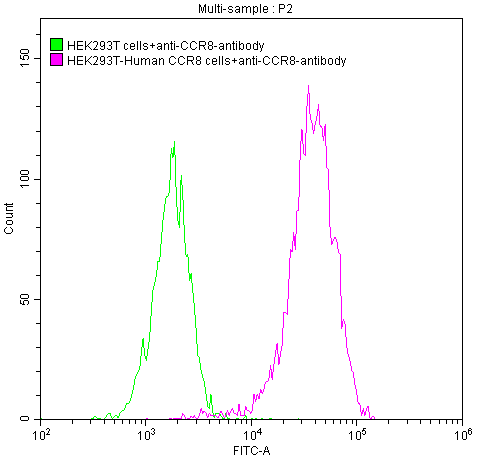
CCR8 Monoclonal Antibody (CSB-RA004847A1HU)
Untransfected HEK293T cells (green line) and transfected Human CCR8 HEK293T stable cells (red line) were stained with anti-CCR8 antibody (2µg/1*106 cells), washed and then followed by FITC-conjugated anti-Human IgG Fc antibody and analyzed with flow cytometry.
● Antibody Activity validation by Functional ELISA
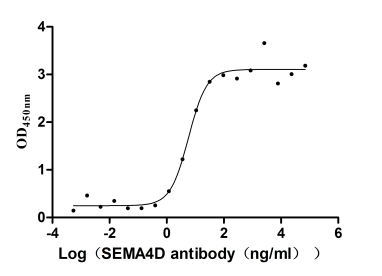
SEMA4D Monoclonal Antibody (CSB-RA835707A1HU)
The Binding Activity of Human SEMA4D with Anti-SEMA4D recombinant antibody.
Activity: Measured by its binding ability in a functional ELISA. Immobilized Human SEMA4D (CSB-MP835707HU) at 2 μg/mL can bind Anti-SEMA4D recombinant antibody, the EC50 is 1.082-4.805 ng/mL.
Product List
| Target |
Product Name |
Species Reactivity |
Tested Applications |
Code |
| ACTB |
ACTB Recombinant Monoclonal Antibody |
Human |
ELISA, WB, FC |
CSB-RA000091M1m |
| ADAM10 |
ADAM10 Recombinant Monoclonal Antibody |
Human |
ELISA, FC |
CSB-RA001273MA1HU |
| AIFM1 |
AIFM1 Recombinant Monoclonal Antibody |
Human |
ELISA, IHC, IF, FC |
CSB-RA001492MA1HU |
| ANPEP |
ANPEP Recombinant Monoclonal Antibody |
Human |
ELISA, IF, FC |
CSB-RA001827MA1HU |
| ANXA1 |
ANXA1 Recombinant Monoclonal Antibody |
Human |
ELISA, IHC, FC |
CSB-RA001836MA1HU |
| ANXA4 |
ANXA4 Recombinant Monoclonal Antibody |
Human, Mouse |
ELISA, WB, FC |
CSB-RA001845MA1HU |
| APP |
APP Recombinant Monoclonal Antibody |
Human |
ELISA, IF, FC |
CSB-RA001950MA3HU |
| ATP7B |
ATP7B Recombinant Monoclonal Antibody |
Human |
ELISA, IF, FC |
CSB-RA002415MA1HU |
| BTLA |
BTLA Recombinant Monoclonal Antibody |
Human |
ELISA, FC |
CSB-RA773799MA1HU |
| C5 |
C5 Recombinant Monoclonal Antibody |
Human |
ELISA, FC |
CSB-RA003995MA1HU |
| CALR |
CALR Recombinant Monoclonal Antibody |
Human, Mouse |
ELISA, WB, IHC, IF, FC |
CSB-RA004458MA1HU |
| CAV1 |
CAV1 Recombinant Monoclonal Antibody |
Human, Mouse, Rat |
ELISA, WB, FC |
CSB-RA004571MA1HU |
| CCR4 |
CCR4 Recombinant Monoclonal Antibody |
Human |
ELISA, FC |
CSB-RA004843MA01HU |
| CCR5 |
CCR5 Recombinant Monoclonal Antibody |
Human |
ELISA, FC |
CSB-RA004844MA1HU |
| CCR8 |
CCR8 Recombinant Monoclonal Antibody |
Human |
FC |
CSB-RA004847A1HU |
| CCR8 |
CCR8 Recombinant Monoclonal Antibody |
Human |
FC |
CSB-RA004847MA3HU |
| CCR8 |
CCR8 Recombinant Monoclonal Antibody |
Human |
FC |
CSB-RA004847MA2HU |
| CCR8 |
CCR8 Recombinant Monoclonal Antibody |
Human |
FC |
CSB-RA004847MA4HU |
| CD147 |
CD147 Recombinant Monoclonal Antibody |
Human |
ELISA, WB, IHC, IF, FC |
CSB-RA002831A0HU |
| CD274 |
CD274 Recombinant Monoclonal Antibody |
Human |
ELISA, IHC, FC |
CSB-RA878942MA1HU |
References
[1] Partridge M.A., Purushothama S., Elango C., et al. Emerging technologies and generic assays for the detection of anti-drug antibodies [J]. J. Immunol. Res. 2016.
[2] Rosenberg A.S., Sauna Z.E. Immunogenicity assessment during the development of protein therapeutics [J]. J. Pharm. Pharmacol. 2018; 70: 584–594.
[3] Pratt KP. Anti-Drug Antibodies: Emerging Approaches to Predict, Reduce or Reverse Biotherapeutic Immunogenicity [J]. Antibodies (Basel). 2018 May 31; 7(2):19.