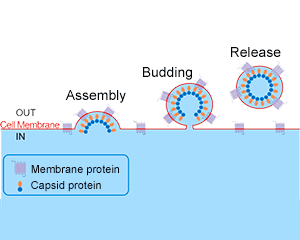
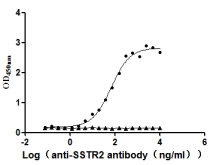
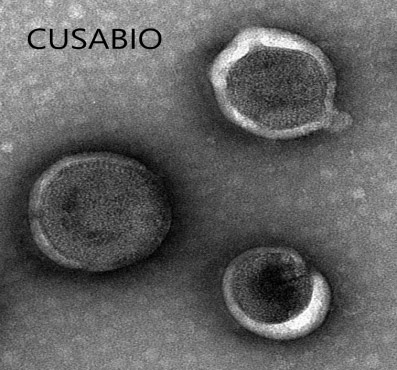
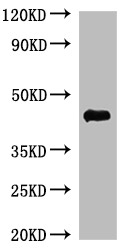
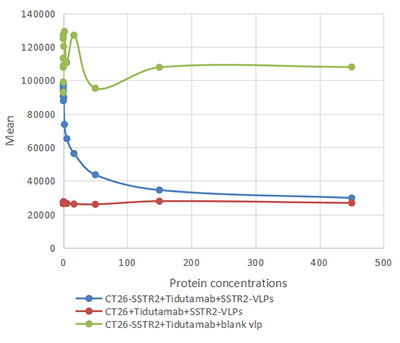
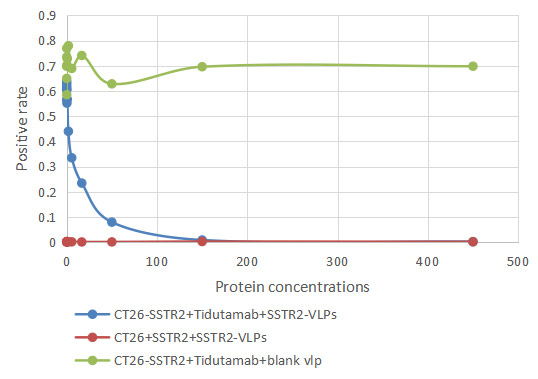
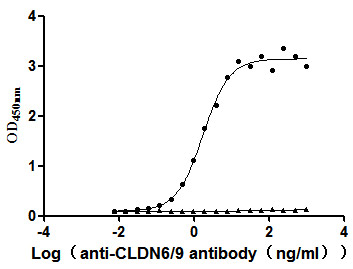
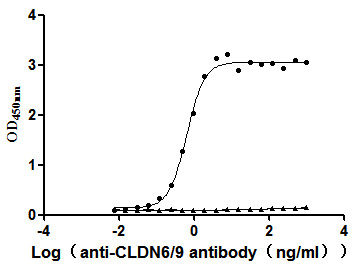
[1] Roldão, A., et al. (2010). Virus-like particles in vaccine development. Expert Review of Vaccines, 9(10), 1149-1176.
[2] Naskalska, A., Pyrc, K. (2015). Virus like particles as immunogens and universal nanocarriers. Polish Journal of Microbiology, 64(1), 3-13.
[3] Lua, L. H., et al. (2014). Bioengineering virus-like particles as vaccines. Biotechnology and Bioengineering, 111(3), 425-440.
[4] Noad, R., & Roy, P. (2003). Virus-like particles as immunogens. Trends in Microbiology, 11(9), 438-444.
[5] Grgacic, E. V., & Anderson, D. A. (2006). Virus-like particles: passport to immune recognition. Methods, 40(1), 60-65.
[6] Vicente, T., et al. (2011). Large-scale production and purification of VLP-based vaccines. Journal of Invertebrate Pathology, 107, S42-S48.
[7] Mena, J. A., & Kamen, A. A. (2011). Insect cell technology is a versatile and robust vaccine manufacturing platform. Expert Review of Vaccines, 10(7), 1063-1081.
[8] Yusibov, V., et al. (2011). Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Human Vaccines, 7(3), 313-321.
[9] Swartz, J. R. (2012). Advances in Escherichia coli production of therapeutic proteins. Current Opinion in Biotechnology, 12(2), 195-201.
[10] Wurm, F. M. (2004). Production of recombinant protein therapeutics in cultivated mammalian cells. Nature Biotechnology, 22(11), 1393-1398.
[11] Bachmann, M. F., & Jennings, G. T. (2010). Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nature Reviews Immunology, 10(11), 787-796.
[12] Plummer, E. M., & Manchester, M. (2011). Viral nanoparticles and virus-like particles: platforms for contemporary vaccine design. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 3(2), 174-196.
[13] Roldão, A., Mellado, M. C. M., Castilho, L. R., Carrondo, M. J. T., & Alves, P. M. (2010). Virus-like particles in vaccine development. Expert Review of Vaccines, 9(10), 1149-1176.
[14] Lua, L. H. L., Connors, N. K., Sainsbury, F., Chuan, Y. P., Wibowo, N., & Middelberg, A. P. J. (2014). Bioengineering virus-like particles as vaccines. Biotechnology and Bioengineering, 111(3), 425-440.
[15] Grgacic, E. V. L., & Anderson, D. A. (2006). Virus-like particles: passport to immune recognition. Methods, 40(1), 60-65.
[16] Brune, K. D., & Howarth, M. (2018). New routes and opportunities for modular construction of particulate vaccines: stick, click, and glue. Frontiers in Immunology, 9, 1432.
[17] Castilho, A., Beihammer, G., Pfeiffer, C., & Goritzer, K. (2018). An in planta-produced fusion protein eliciting robust antibody response against the major grass pollen allergens Phl p 1 and Phl p 5a. Plant Biotechnology Journal, 16(10), 1736-1745.
[18] Noad, R., & Roy, P. (2003). Virus-like particles as immunogens. Trends in Microbiology, 11(9), 438-444.
[19] Schwarz, B., & Douglas, T. (2015). Development of virus-like particles for diagnostic and prophylactic biomedical applications. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 7(5), 722-735.
[20] Chroboczek, J., Szurgot, I., & Szolajska, E. (2014). Virus-like particles as vaccine. Acta Biochimica Polonica, 61(3), 531-539.