CUSABIO Therapeutic Antibody Discovery Service
Antibody drugs have fewer and fewer adverse reactions because of
their high
specificity. Therefore, therapeutic antibody has become the predominant class of new drugs developed in
recent
years. Basically, the whole research and development process of antibody drugs will take 10 years, from
the
initial identification of targets, to screening of therapeutic antibody candidates for pre-clinical
studies, to
enter clinical phase Ⅰ/ Ⅱ / Ⅲ trials, and then to complete the final declaration of NDA (new drug
application).
Throughout the whole process of antibody drug development, therapeutic antibody discovery service is the
very
beginning of antibody-based drug discovery, and it is crucial as well.
CUSABIO has been working on the development and production of
proteins and
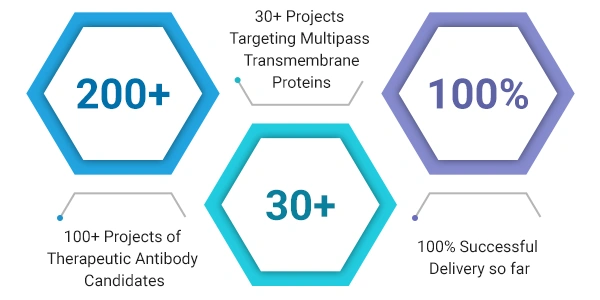
antibodies for more than 18 years and successfully screened 200+ therapeutic antibody candidates for
pharmaceutical companies already, many of which have entered the CMC or IND phases. For some challenging
targets, including CLDN18.2,
CLDN6,
CCR8,
SSTR2,
DLL3,
LY6G6D,
ROR1,
SEMA4D,
GPC3,
CD16A
and so on, we not only successfully had produced antigens, but also successfully screened corresponding
recombinant antibodies already. CUSABIO has 3 platforms for production of transmembrane proteins. Mostly,
we
have completed 30+ projects of screening services targeting multi-pass transmembrane proteins. So we can
confidently promise that CUSABIO has rich experiences in therapeutic antibody discovery service targeting
multi-pass transmembrane protein, and we can offer
the
therapeutic antibody discovery service to you as risk-free, we can promise that we will not charge any fee
if we
failed in screening.
CUSABIO can provide a one-stop solution from target to therapeutic
antibody
candidate to accelerate the entire development process for you.