ADC Development Solutions
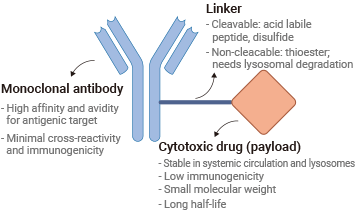
Antibody-drug conjugates (ADCs) are a class of targeted therapies designed to deliver powerful drugs directly to cancer cells. They combine the precision of monoclonal antibodies with the potency of cytotoxic drugs, creating a highly effective approach to treatment while minimizing damage to healthy tissues.
An ADC consists of three key components:
- Antibody, a lab-engineered protein that specifically binds to cancer cell markers.
- Linker, a chemical bridge that attaches the drug to the antibody and controls its release.
- Payload, a potent drug that kills cancer cells once delivered inside.
When an ADC binds to its target on a cancer cell, it is absorbed and broken down, releasing the drug exactly where it's needed. This targeted approach improves treatment precision, reduces side effects, and enhances the therapeutic impact.
The development of antibody-drug conjugates (ADCs) is a complex, multi-stage process that typically involves four key phases: Discovery, Design and Synthesis, Preclinical Development, and Clinical Research.
With extensive experience in protein, antibody, and ELISA kit development, CUSABIO provides tailored products, services, and solutions to support early-stage research & development, as well as clinical and preclinical studies.
Early-stage Research & Development
ADCs Workflow steps
Products & services that CUSABIO offers
Antigen Preparation, Validation & Immunization
Antibody Molecular Discovery
Antibody Molecular Screening & Optimization
Clinical & Preclinical Studies
Pharmacokinetic Analysis & Immunogenicity Analysis
No time to go through the information below? Just let us know your requirements, and our experts will provide a solution within 12 hours.
ADCs Target Protein and Customization Services
In the early stages of ADC drug development, the antibody must specifically bind to antigens on tumor cells to target them accurately. As a result, preparing highly pure and active target antigen proteins is essential for successful ADC development. These proteins are used to screen and validate antibody specificity, assess ADC drug cytotoxicity in vitro, and perform cell function tests.
CUSABIO has years of experience in protein expression and offers a range of target antigens from different species and with various tags. These proteins are suitable for experiments like immunization, antibody screening, SPR, and cell activity assays.
Here are the key features of these proteins.
● Multi-species Proteins for Cross-validation
B-lymphocyte antigen CD20 (MS4A1)
Species: Macaca fascicularis
● High Purity
● ELISA/LSPR/BIL for Bioactivity Verification
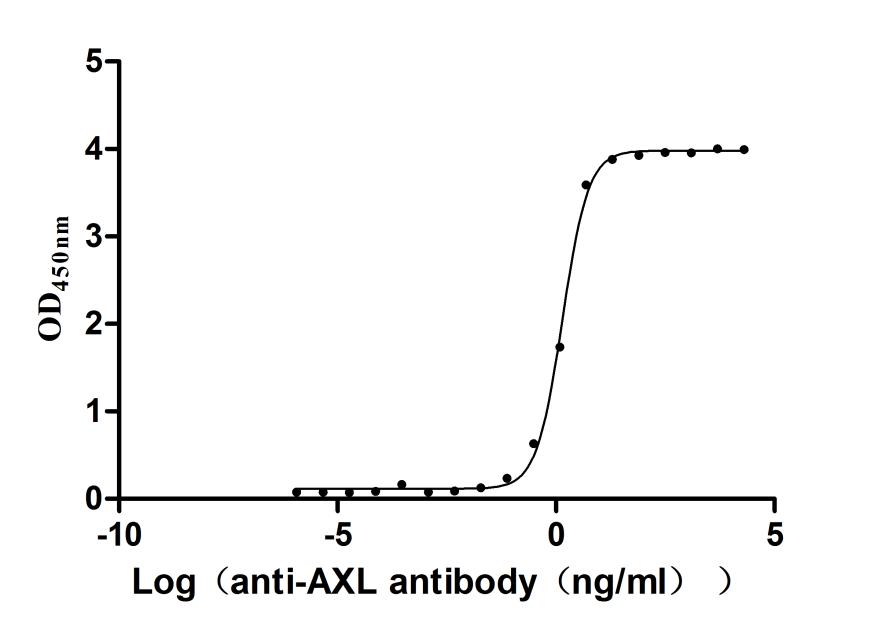
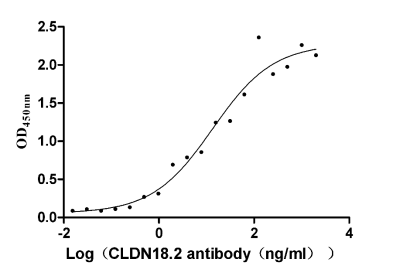
Recombinant Human Claudin-18.2 (CLDN18.2)
CSB-MP005498HU(A5)l5
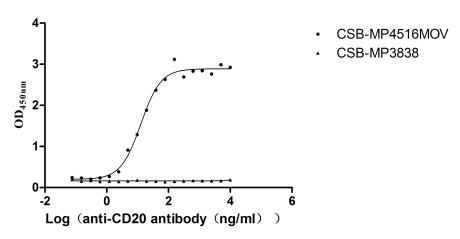
Measured by its binding ability in a functional ELISA. Immobilized Human CLDN18.2 at 10 μg/ml can bind Anti-CLDN18.2 recombinant antibody (CSB-RA005498A1HU), the EC50 is 5.115-8.534 ng/mL. The VLPs (CSB-MP3838) are negative control.
Recombinant Human Leukocyte surface antigen CD47 (CD47)
CSB-MP004940HU
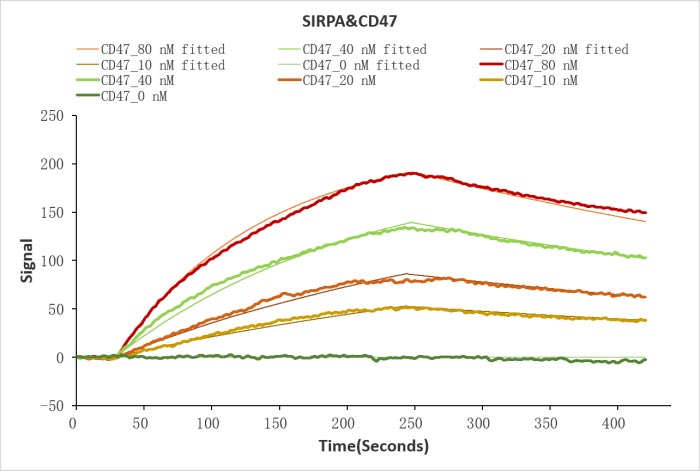
Human SIRPA protein His/Myc tag (CSB-MP021334HU) captured on COOH chip can bind Human CD47 protein Fc tag (CSB-MP004940HU) with an affinity constant of 19.1 nM as detected by LSPR Assay.
Recombinant Human Cystine/glutamate transporter (SLC7A11)
CSB-CF892171HU(A4)
Loaded Anti-Human SLC7A11 Antibody (CSB-RA892171MA1HU) on 96-Flat plate, can bind Human SLC7A11, with an affinity constant of <1 pM as determined in BLI assay (Gator Prime).
● High Batch-to-Batch Consistency
● ADCs Target Protein Products
● ADCs Target Protein Customization Service
Therapeutic Antibody Discovery Service
Antibody discovery is the first and critical step in drug development, directly impacting efficacy and safety. For 17 years, CUSABIO has focused on protein and antibody R&D, screening over 100 therapeutic antibody candidates for pharmaceutical companies—many now in the CMC or IND stages. With expertise in antigen preparation and antibody screening, we provide a one-stop solution from target identification to therapeutic antibody candidates, helping accelerate drug development.
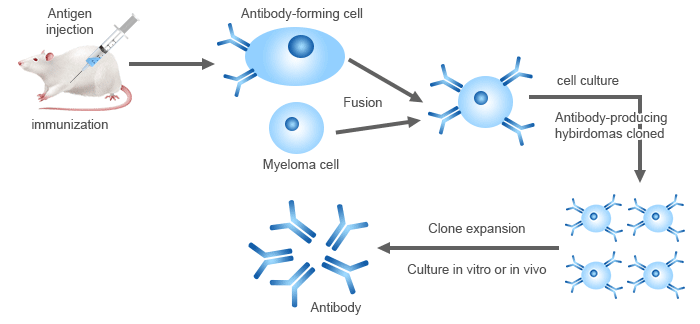
CUSABIO offers two antibody discovery platforms: hybridoma and single B cell technology.
● Hybridoma Antibody Discovery
CUSABIO has 16 years of experience in hybridoma monoclonal antibody development. Their technical processes are mature, ensuring stable and reliable product quality. The initial positive rate reaches 20%-40%, while the FACS positive rate is 10%-20%. These rates meet the diverse needs of various customers.
CUSABIO performs functional tests on therapeutic antibodies after screening. These tests include antigen ELISA, cell FACS, antigen SPR, and antigen-binding assays. The tests are tailored to your needs. They ensure the antibodies work well in FACS experiments with stable cell lines.
- Case Display
ELISA preliminary screening after electrofusion
FACS screening of supernatants after subcloning

18 positive clones from different plates were determined after comparison with the reference antibody.
Recombinant expression after sequencing positive clones

According to the FACS ranking, the top 7 unique sequences were expressed and validated with the A549 cell.
● Single B Cell Antibody Discovery
Single B cell antibody technology has several advantages over traditional methods like hybridoma and phage display. It has shorter development cycles, higher throughput, more positive clones, and higher affinity, ensuring the natural pairing of light and heavy chains.
CUSABIO provides a one-stop service for single B cell antibody preparation. The service is fast, high-throughput, and delivers antibodies with high specificity and affinity. Our comprehensive process includes immunogen design, antibody production, and detection method development, ensuring clients receive high-quality antibody solutions.
- Case Display
Linear expression vector construction
Antibody Molecular Optimization
The mammalian cell display platform is designed to optimize therapeutic antibodies' developability. Mammalian cells grown in dishes closely resemble human clinical conditions and industrial antibody production. This platform is a cutting-edge tool for antibody engineering, including affinity maturation and humanization.
● CUSABIO Presents:
Fine-tuned biophysical property
Best for antibody engineering
- Application 1: Affinity
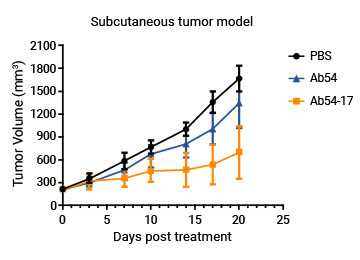
The affinity of the customer's mAb was enhanced 100-fold by mutating one AA in CDR3.
| Target |
Analyte |
Kon (1/Ms) |
Koff (1/s) |
KD (M) |
RU2 |
| Confidential_1 |
Ab54 (WT) |
3.76E+05 |
1.24E-02 |
3.30E-08 |
0.26 |
| Ab54-17 |
3.83E+05 |
1.06E-04 |
2.77E-10 |
0.97 |
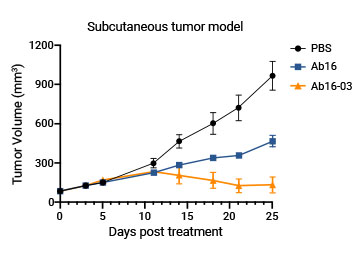
The affinity of the customer's mAb against cynomolgus antigen was enhanced 100-fold by mutating one AA in each CDR3 while retaining the affinity to human antigen.
| Target |
Analyte |
Kon (1/Ms) |
Koff (1/s) |
KD (M) |
RU2 |
| Confidential_2 (Human) |
Ab16 (WT) |
4.13E+05 |
1.85E-04 |
4.48E-10 |
3.44 |
| Ab16-03 |
4.53E+05 |
2.09E-04 |
4.61E-10 |
1.79 |
| Confidential_2 (Cynomolgus) |
Ab16 (WT) |
1.81E+05 |
5.24E-03 |
2.90E-08 |
1.87 |
| Ab16-03 |
8.92E+05 |
4.47E-04 |
5.01E-10 |
4.32 |
Customers' in vivo data further proves the good quality of the engineered variants.
More affinity maturation
Blocking activity of small molecules (chemical compound)
- Application 2: Humanization
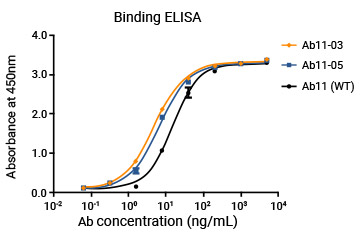
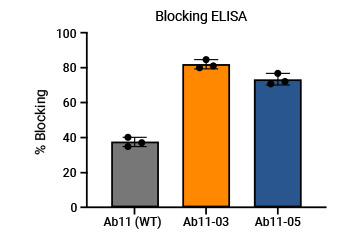
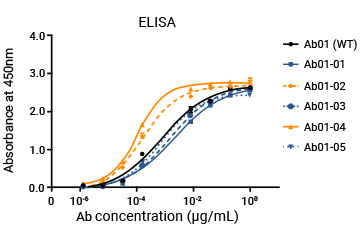
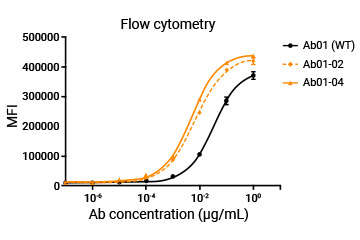
The customer's mAb was humanized through a library specifically designed for screening high-affinity molecules, and the affinity of humanized antibodies could be 10-fold higher than the parent molecule.
| Target |
Analyte |
Kon (1/Ms) |
Koff (1/s) |
KD (M) |
RU2 |
| Confidential_3 |
Ab01 (WT) |
4.06E+04 |
3.11E-05 |
7.66E-10 |
0.45 |
| Ab01-01 |
2.17E+04 |
7.06E-06 |
3.25E-10 |
1.11 |
| Ab01-02 |
7.22E+04 |
7.49E-06 |
1.04E-10 |
0.14 |
| Ab01-03 |
5.61E+04 |
4.78E-05 |
8.52E-10 |
2.76 |
| Ab01-04 |
2.79E+04 |
2.38E-06 |
8.53E-11 |
2.59 |
| Ab01-05 |
4.89E+04 |
4.25E-05 |
8.69E-10 |
3.73 |
WT and the engineered molecules were also compared through ELISA and flow cytometry.
Positive Control Antibody
CUSABIO supports drug development by developing recombinant antibodies for drug discovery. These recombinant antibodies are expressed in mammalian cells and tested in protein activity assays. They can serve as positive controls in drug discovery or validate the activity of corresponding proteins.
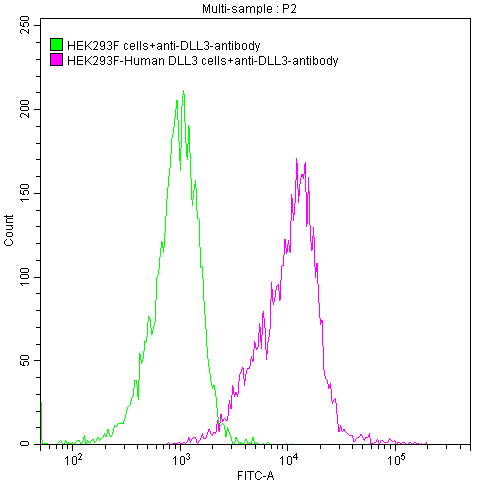
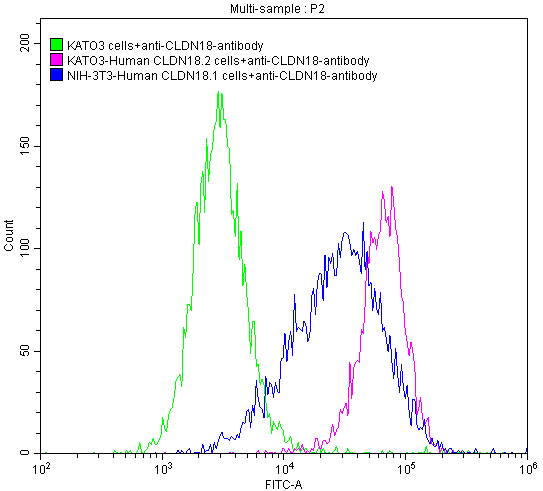
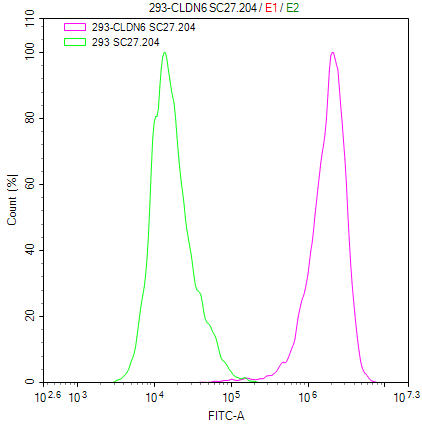
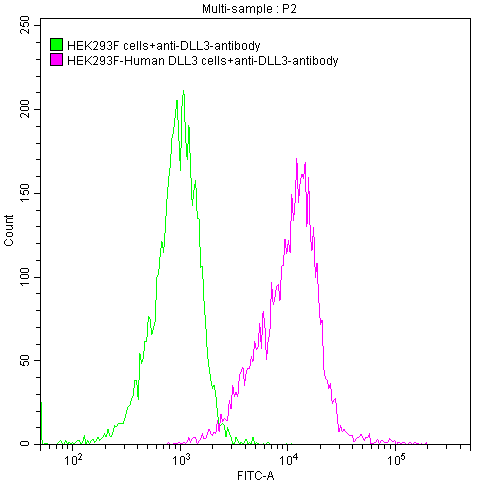
FC Validation of Antibody Specificity
DLL3 Recombinant Monoclonal Antibody
CLDN18 Recombinant Monoclonal Antibody
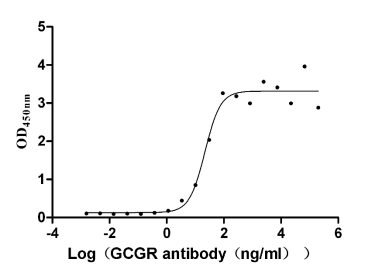
Functional ELISA for Validating Antibody Activity
GCGR Recombinant Monoclonal Antibody
Claudin-18.2 Recombinant Monoclonal Antibody
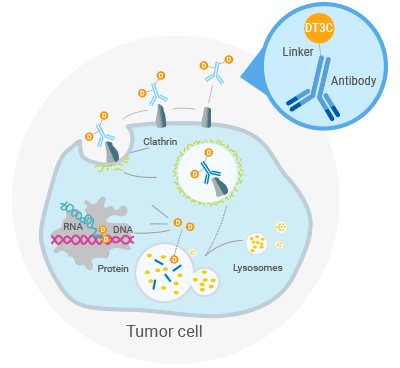
DT3C Recombinant Protein
DT3C is a recombinant protein mainly used for in vitro evaluation of the internalization efficiency of cancer cells towards monoclonal antibodies (mAbs), thereby enabling the screening of more effective monoclonal antibodies. This protein contains a diphtheria toxin (DT) protein lacking a receptor binding domain and Streptococcus protein G.
Compared to commonly used antibody internalization efficiency detection techniques such as acid buffer elution, Mab-ZAP, pHrodo, etc., the DT3C recombinant protein assay is characterized by its simplicity, speed, and high detection efficiency.
Simple preparation: mAb-DT3C conjugates can be formed by incubating at room temperature for 30 minutes.
Multi-species applicability: It can be combined with any IgG from different species to form mAb-DT3C conjugates.
Rapid evaluation: The internalization efficiency of mAb on cell surfaces can be quickly and accurately detected using techniques such as flow cytometry or fluorescence microscopy.
High internalization efficiency: The internalization efficiency of antibodies is significantly higher than that of Mab-ZAP.
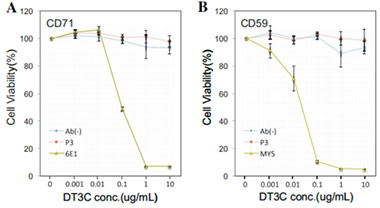
In vitro detection of ADC antibody internalization efficiency
Antibody internalization efficiency is higher than Mab-ZAP
In vitro detection of ADC antibody internalization efficiency
Reference: M. Yamaguchi et al. Biochemical and Biophysical Research Communications 454 (2014) 600-603.
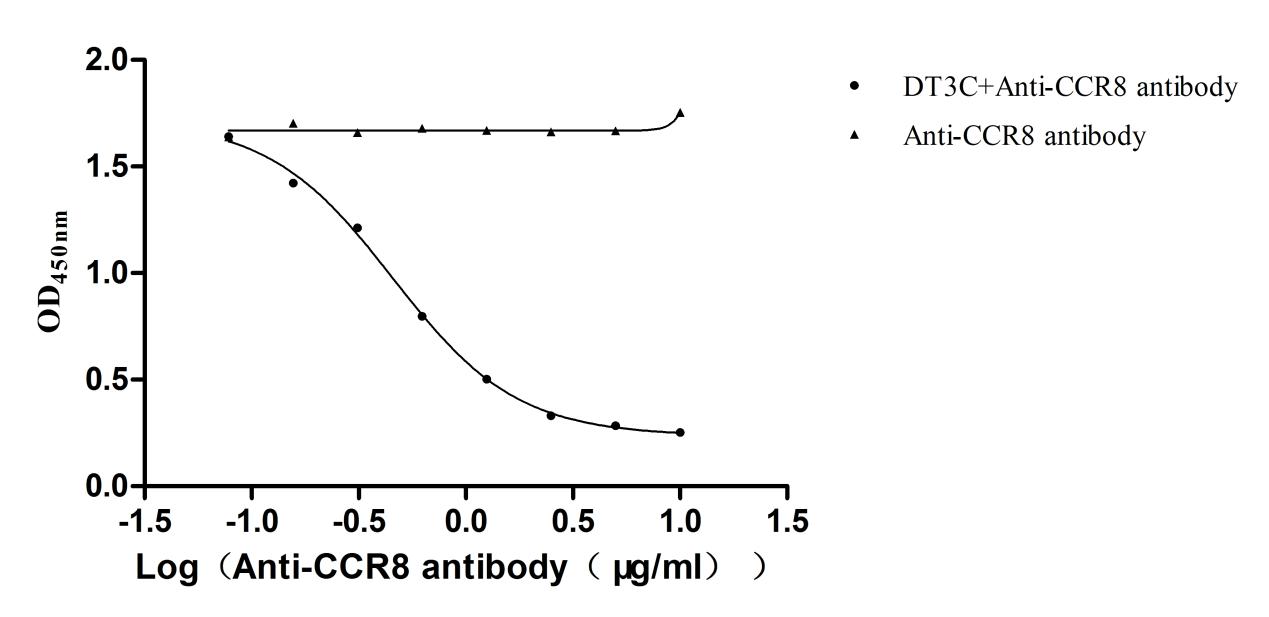
DT3C and CCR8 antibodies prepared by CUSABIO are incubated and then applied to different cell lines
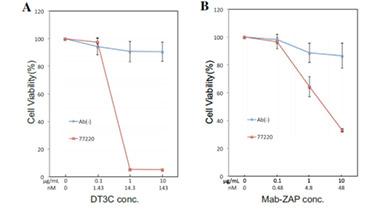
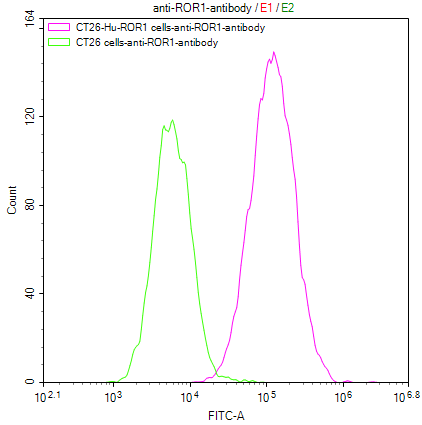
After DT3C 10μg/ml formed complexes with different concentrations of Anti-CCR8 antibodies (CSB-RA004847MA3HU), cytotoxicity experiments were conducted on CHOK1/Human CCR8 cells (CSB-SC004847HU2). The ED50 is 0.3708-0.5608 μg/ml.
After DT3C 10μg/ml formed complexes with different concentrations of Anti-CCR8 antibodies (CSB-RA004847MA3HU), cytotoxicity experiments were conducted on CT26/Human CCR8 cells (CSB-SC004847HU3). The ED50 is 0.4661-0.8614 μg/ml.
Overexpression Cell Line and Construction Service
Overexpression stable cell lines are specially engineered cells generated from a specific cellular lineage that can stably overexpress or suppress a particular gene. Unlike transient transfection, which only allows transient gene expression, these cell lines can continue to express specific genes.
● Applications
Disease Model Establishment
Small Molecule and Antibody Drug Development
● Service Advantages
Mature Stable Cell Line Development Platform
Equipped with comprehensive service platforms for cells, antibodies, proteins, etc., it can provide one-stop service from stable cell line construction to recombinant protein production.
Strict Quality Control System
Double detection of microorganisms such as bacteria and mycoplasma, ensuring 100% contamination-free and cell viability testing is conducted to guarantee delivery quality.
Stable Cell Passage
High-quality delivery standards ensure stable passage of cells for more than 20 generations.
Professional Technical Support
Professional technical personnel regularly report on project progress to ensure the smooth progress of the project.
Our Commitment:No charges if it doesn't pass the flow cytometry detection.
● Case Display
Reporter Gene Cell Line and Construction Service
Reporter gene cell line construction uses molecular cloning techniques. This process inserts a reporter gene (e.g., luciferase) into cellular DNA. Subsequent selection and cultivation establish stable cell lines with continuous reporter gene expression.
● Applications
ADCC/ADCP Functional Validation
Protein Interaction (Signaling Pathway) Research
Evaluation of Cross-linking Drugs
CUSABIO provides reporter gene cell line construction services and spot products.
● Case Display
Anti-payload Antibodies and Customized Service
Payload is a key component of ADC drugs. It targets tumor cell molecules to disrupt their functions and prevent growth and division, thus killing cancer cells. During ADC quality control, anti-payload antibodies are used to detect the ADC's payload, evaluating drug stability, safety, and efficacy.
CUSABIO provides research tools for preclinical and clinical studies of anti-payload antibodies (e.g., Anti-DXD mAb, Anti-MMAE/MMAF, Anti-Eribulin, etc.). These tools enable pharmacokinetic monitoring and drug-antibody ratio (DAR) analysis in blood samples.
● Types of Anti-payload Antibodies
● Case Display
High Purity
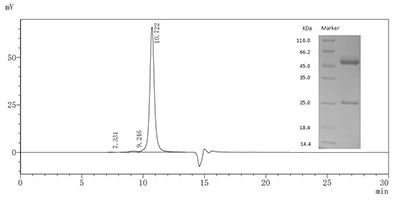
The Mouse Anti-DXD Antibody (Code: CSB-MA996977I2m) has a purity of more than 90% as determined by SEC-HPLC and SDS-PAGE.
High Purity
The Mouse Anti-Eribulin Antibody (Code: CSB-MA943989I1m) has a purity of more than 98% as determined by SEC-HPLC and SDS-PAGE.
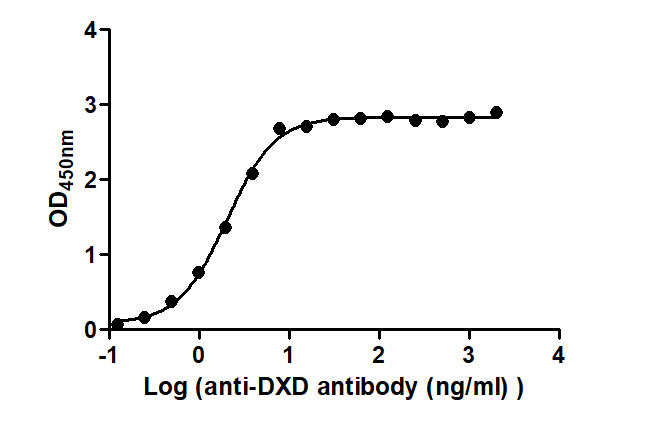
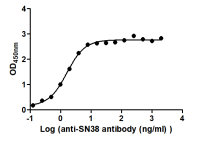
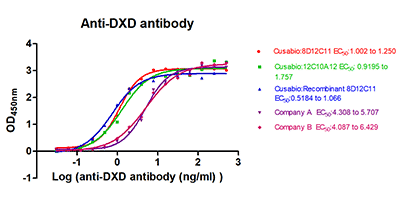
High-Affinity Functional ELISA Validation
High Affinity for Clinical ADC Drugs
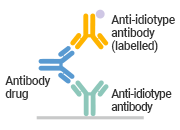
Anti-idiotype Antibody Customization Service
Anti-idiotype antibodies share structural similarities with anti-drug antibodies (ADAs), which enables their use as positive controls for ADA quantification in immunogenicity assessments. In pharmacokinetic (PK) and pharmacodynamic (PD) studies, these antibodies are used to measure blood levels of antibody drugs in free, bound, and total forms.
CUSABIO provides anti-idiotypic rabbit polyclonal antibody preparation and anti-idiotypic mouse mAb preparation (anti-Fab and anti-ScFv). Leveraging five core protein expression and kit development platforms, CUSABIO delivers end-to-end solutions encompassing antigen production, anti-idiotype antibody development, analytical assay establishment, and kit development.
| Service type |
Anti-idiotypic mouse mAb preparation
- Anti-Fab mouse mAb
- Anti-ScFv mouse mAb
|
Anti-idiotypic rabbit polyclonal antibody preparation |
| Main application |
PK analysis |
Immunogenicity analysis |
| Service period |
16-26 weeks |
5-11 weeks |
| Advantages |
Better specificity, but immune originality evaluation results are lower than anti-idiotypic rabbit polyclonal antibodies. |
Can better simulate the real situation of test subject blood samples, but specificity is lower than anti-idiotypic mouse mAbs. |
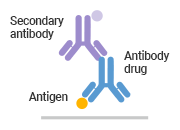
● Comparison of Different Methods in Pharmacokinetic (PK) Analysis
| Type |
Antigen Capture |
Anti-idiotype Antibody Capture |
Sandwich ELISA |
| Schematic Diagram |
|
|
|
| Advantages |
Anti-idiotypic antibodies are not required |
Only one anti-idiotypic antibody is needed, a relatively simple development process |
High sensitivity; high accuracy |
| Disadvantages |
Lower sensitivity; lower accuracy |
Easy to have cross-reactions with other subtypes |
Relatively difficult development process |
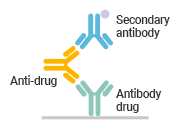
● Comparison of Different Methods in Immunogenicity Analysis
| Type |
Antigen Capture |
Anti-idiotype Antibody Capture |
| Schematic Diagram |
|
|
| Advantages |
Anti-idiotypic antibodies are not required |
High accuracy |
| Disadvantages |
Lower sensitivity; lower accuracy |
Relatively difficult development process, limited detection range |
Cytokine ELISA Kits & Kit Customization Service
Cytokine detection is critical for ADC drug development, particularly in the immunogenicity assessment, pharmacodynamics studies, and safety evaluations.
CUSABIO has developed the IS series of cytokine detection assay kits for industrial clients, including pharmaceutical companies. These kits are produced with high-performance raw materials, meet strict production standards, and undergo multiple quality controls. Developed through advanced R&D platforms, these kits are suitable for key applications: cytokine release syndrome (CRS) detection, residual process material testing in drug manufacturing, pharmacokinetic experimental cell function evaluation, quality control in developing cell therapy drugs, and pharmacological efficacy testing.
● IS series VS RUO series
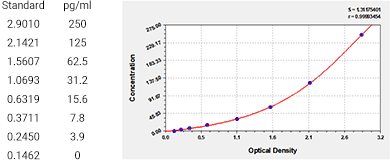
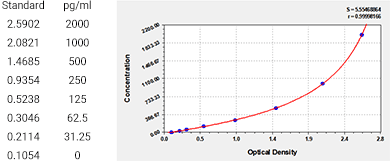
Human Interleukin 10,IL-10 ELISA KIT as an example:
IS series industrial special products--CSB-E04593h-IS
Sample Types: serum, plasma, tissue homogenates, cell culture supernates
Detection Range: 3.9 pg/ml-250 pg/ml
Sensitivity: 0.975 pg/ml
Standard Curve:
Precision:
|
Intra-Assay Precision |
Inter-Assay Precision |
| Sample |
1 |
2 |
3 |
1 |
2 |
3 |
| n |
20 |
20 |
20 |
20 |
20 |
20 |
Mean
(pg/ml) |
35.83 |
33.975 |
36.452 |
33.565 |
36.973 |
36.037 |
| SD |
0.031 |
0.024 |
0.029 |
0.041 |
0.034 |
0.036 |
| CV(%) |
5.688 |
4.554 |
5.263 |
7.839 |
6.115 |
6.581 |
Conventional scientific research products--CSB-E04593h
Sample Types: serum,plasma, tissue homogenates
Detection Range: 31.25 pg/ml- 2000 pg/ml
Sensitivity: 7.8 pg/ml
Standard Curve:
Precision:
|
Intra-Assay Precision |
Inter-Assay Precision |
| Sample |
1 |
2 |
3 |
1 |
2 |
3 |
| n |
20 |
20 |
20 |
20 |
20 |
20 |
Mean
(pg/ml) |
251.241 |
254.176 |
249.776 |
246.854 |
255.646 |
251.974 |
| SD |
0.028 |
0.024 |
0.027 |
0.04 |
0.037 |
0.035 |
| CV(%) |
5.283 |
4.494 |
5.114 |
7.634 |
6.903 |
6.591 |
● Product List of IS Series Cytokine ELISA Kits
| Product Name |
Code |
Product Name |
Code |
| Human Brain derived neurotrophic factor, BDNF ELISA Kit |
CSB-E04501h-IS |
Human Interleukin 22(IL-22) ELISA KIT |
CSB-E13418h-IS |
| Human glycoprotein 130,gp130 ELISA KIT |
CSB-E04571h-IS |
Mouse Interleukin 22(IL-22) ELISA Kit |
CSB-E13513m-IS |
| Human glycoprotein (transmembrane) nmb (GPNMB) ELISA kit |
CSB-EL009727HU-IS |
Mouse Interleukin 4,IL-4 ELISA KIT |
CSB-E04634m-IS |
| Human hepatocyte growth factor, HGF ELISA kit |
CSB-E04573h-IS |
Mouse Interleukin 6,IL-6 ELISA Kit |
CSB-E04639m-IS |
| Mouse Interferon γ, IFN-γ ELISA Kit |
CSB-E04578m-IS |
Human Interleukin-7,IL-7 ELISA kit |
CSB-E14032h-IS |
| Human Interferon γ (IFN-γ) ELISA Kit |
CSB-E04577h-IS |
Mouse Interleukin-7,IL-7 ELISA kit |
CSB-E10257m-IS |
| Mouse interleukin 10,IL-10 ELISA KIT |
CSB-E04594m-IS |
Mouse Insulin, INS ELISAKit |
CSB-E05071m-IS |
| Human Interleukin 10,IL-10 ELISA Kit |
CSB-E04593h-IS |
Mouse Kidney injury molecule 1, Kim-1 ELISA Kit |
CSB-E08181h-IS |
| Mouse Interleukin 12,IL-12/P70 ELISA KIT |
CSB-E04600m-IS |
Mouse Kidney injury molecule 1, Kim-1 ELISA Kit |
CSB-E08809m-IS |
| Human Interleukin 12, IL-12/P70 ELISA Kit |
CSB-E04599h-IS |
Human Macrophage Inflammatory Protein-1α,MIP-1α ELISA kit |
CSB-E04662h-IS |
| Human Interleukin 13, IL-13 ELISA KIT |
CSB-E04601h-IS |
Human Receptor for advanced glycation end products, RAGE/AGER ELISA Kit |
CSB-E09354h-IS |
| Mouse Interleukin 17, IL-17 ELISA Kit |
CSB-E04608m-IS |
Human Transforming Growth factor β1,TGF-β1 ELISA kit |
CSB-E04725h-IS |
| Human Interleukin 17A (IL-17A/IL-17) ELISA Kit |
CSB-E12819h-IS |
Human Tumor necrosis factor soluble receptor I, TNFsR-I ELISA KIT |
CSB-E04736h-IS |
| Human Interleukin 1β,IL-1βELISA Kit |
CSB-E08053h-IS |
Rat TNF-α ELISA kit |
CSB-E11987r-IS |
| Mouse Interleukin 1β,IL-1β ELISA Kit |
CSB-E08054m-IS |
Mouse Tumor necrosis factor α, TNF-α ELISA Kit |
CSB-E04741m-IS |
| Mouse Interleukn 2,IL-2 ELISA kit |
CSB-E04627m-IS |
Human Tumor necrosis factor α, TNF-α ELISA KIT |
CSB-E04740h-IS |
Currently, CUSABIO provides 287 types of high-quality cell cytokine detection ELISA kits and offers custom antibody pairs and ELISA kit services based on your needs.
Have questions? Our experts are ready to help.

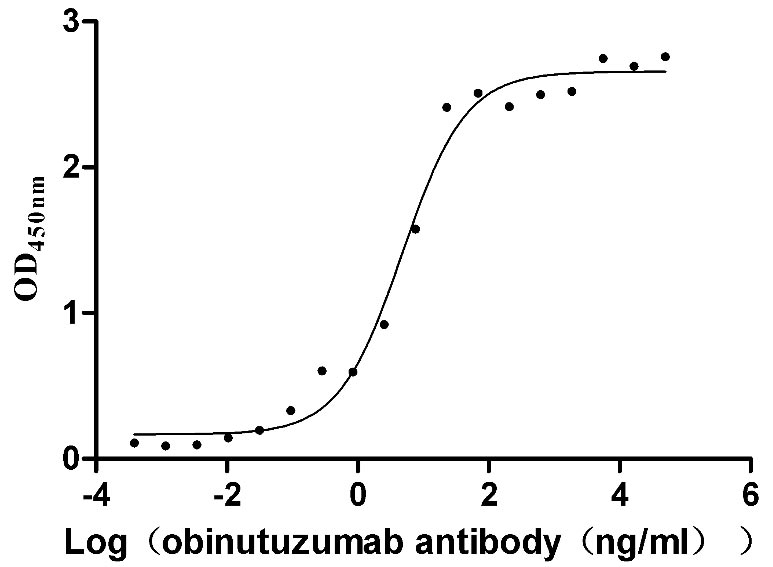
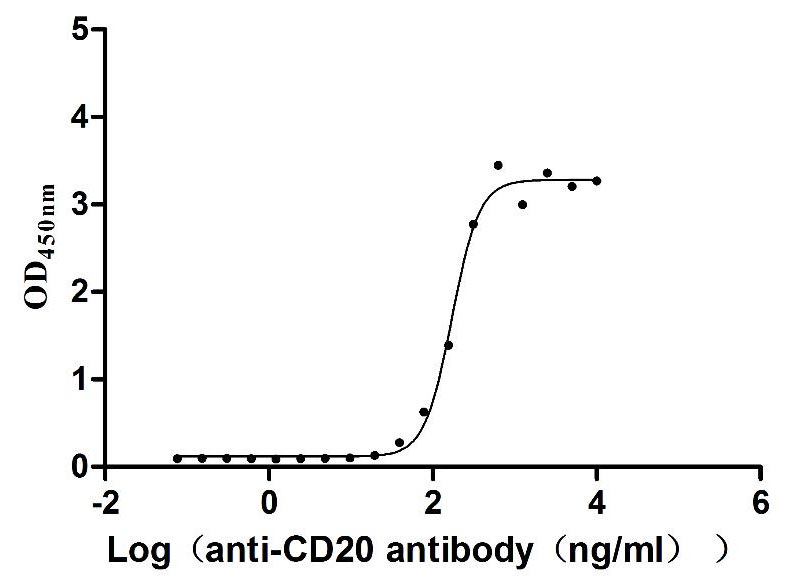

l5-AC1.jpg)
-AC2.jpg)