Stable Cell Line Development Service
The construction of stable cell lines involves the introduction of exogenous genes into recipient cells, where they integrate into the cellular chromosome, enabling the sustained and stable expression of the target antigen on the host cell membrane. Stable cell lines play a pivotal role in biological research, widely employed in applications such as the production of recombinant proteins and monoclonal antibodies, drug screening, and gene function studies.
With years of expertise in protein expression research, CUSABIO possesses extensive experience in the development of stable cell lines. We are proficient in enhancing the production capacity of stable cell lines, thereby improving experimental outcomes. CUSABIO provides a comprehensive, one-stop service from gene sequencing to the delivery of stable overexpression cell lines and reporter gene cell lines, emphasizing our commitment to delivering high-quality solutions for our clients.
Overexpression stable cell lines refer to cell lines generated from a specific cellular lineage with the capability for sustained overexpression or interference of a particular gene. Their defining characteristic lies in their ability to continuously express specific genes, in contrast to transient transfection, which allows only transient expression.
Applications:
- Gene Function Studies
- Drug Screening
- Disease Model Establishment
- Small Molecule and Antibody Drug Development
Reporter gene cell line construction involves the molecular biology cloning technique, where a specific reporter gene (e.g., luciferase) is inserted into the chromosome of the target cells. Through selection and cultivation processes, a cell line capable of stably expressing the reporter gene is obtained.
Applications:
- Gene Expression Regulation Studies
- ADCC/ADCP Functional Validation
- Protein Interaction (Signaling Pathway) Research
- Evaluation of Cross-Linking Drugs
Service Advantages:
Mature Stable Cell Line Development Platform
Equipped with comprehensive service platforms for cells, antibodies, proteins, etc., it can provide one-stop service from stable cell line construction to recombinant protein production.
Strict Quality Control System
Double detection of microorganisms such as bacteria and mycoplasma, ensuring 100% contamination-free, and cell viability testing is conducted to guarantee delivery quality.
Stable Cell Passage
High quality delivery standards ensure stable passage of cells for more than 20 generations.
Professional Technical Support
Professional technical personnel regularly report on project progress to ensure smooth progress of the project.
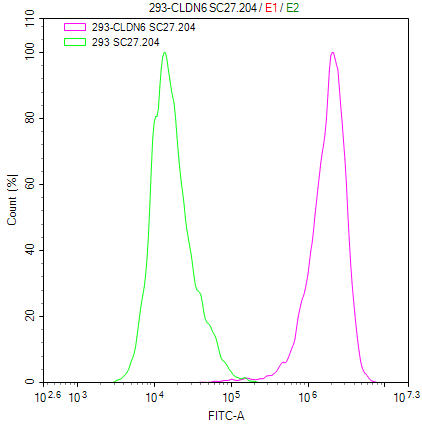
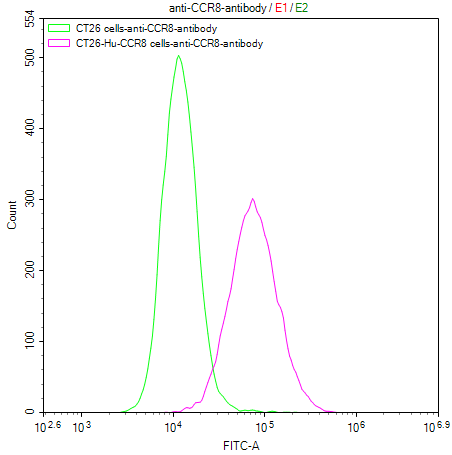
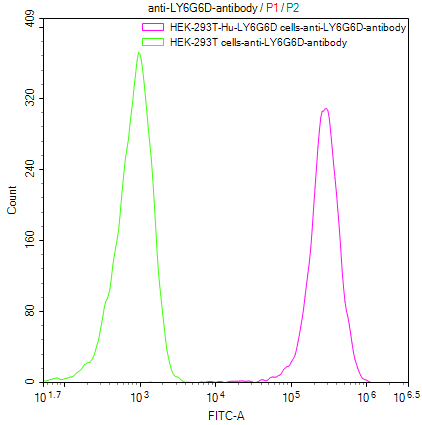
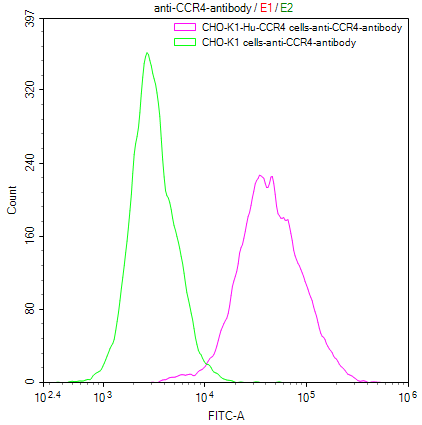
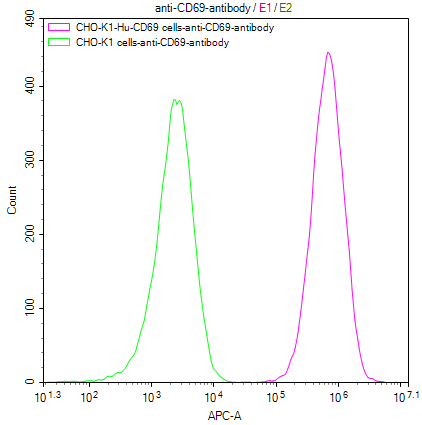
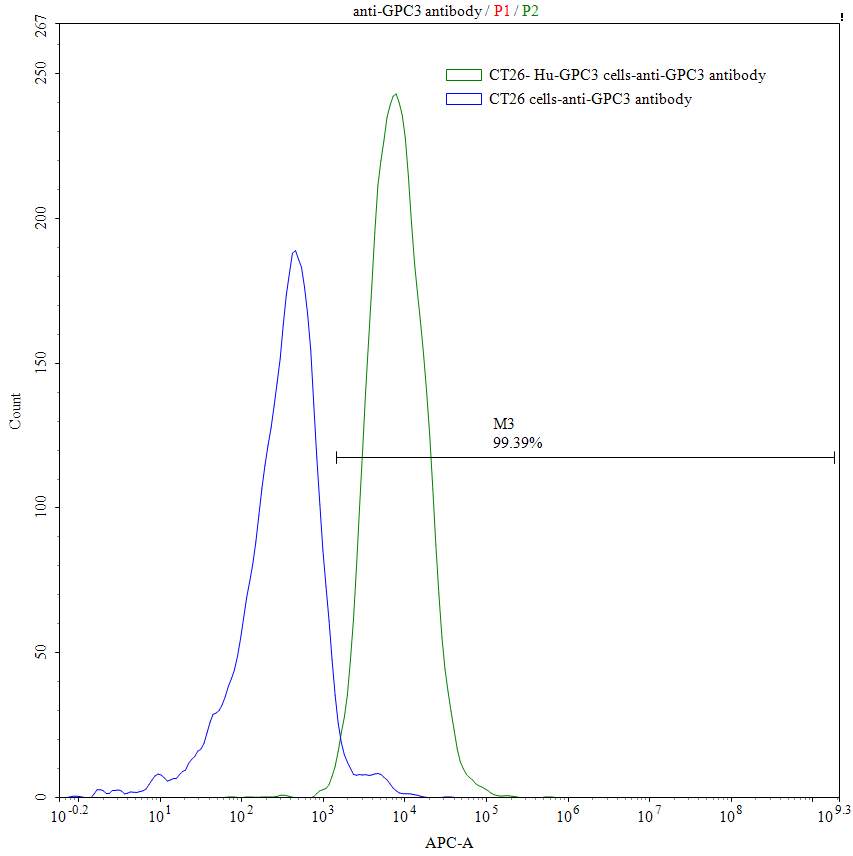
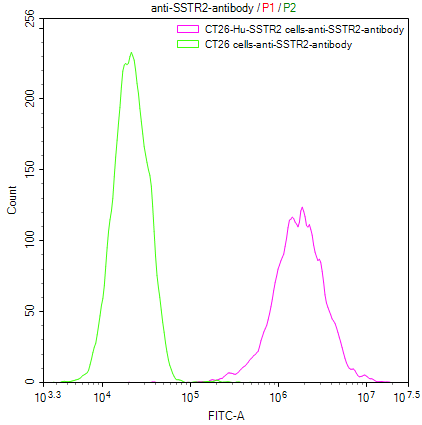
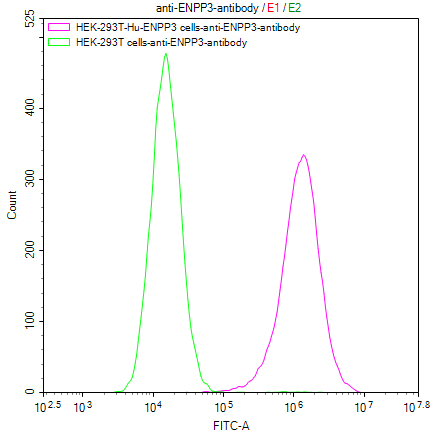
Our Commitment:No charges if it doesn't pass the flow cytometry detection.
Service content and cycle:
Overexpression Cell Line Development
(Total 8-14 Weeks)
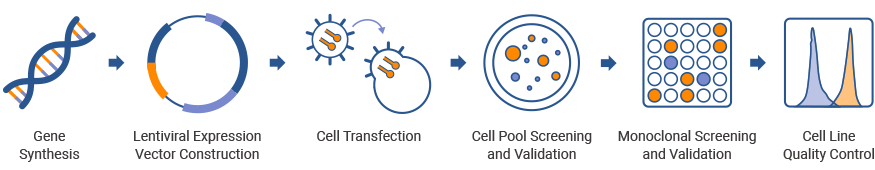
Service Workflow
I
Host Cell Detection (1-2 Weeks)
- Cell viability assessment.
- Cell count measurement.
- Bacteria and fungi detection.
- Mycoplasma detection.
II
Lentiviral Expression Plasmid Construction (2-3 Weeks)
- Codon optimization.
- Whole gene synthesis.
- Construction of lentiviral expression plasmid.
III
Lentivirus Packaging (1-2 Weeks)
- Co-transfection of expression plasmid and helper plasmids into host cells for lentivirus packaging.
IV
Cell Pool Screening and Validation (1-2 Weeks)
- Positive cell selection.
- Measurement of cell pool expression levels.
V
Monoclonal Screening and Validation (2-3 Weeks)
- Single-cell plating and validation of monoclonal expression.
VI
Cell Line Quality Control (1-2 Weeks)
- Expansion cultivation of monoclonals, cryopreservation.
- Recovery viability assessment.
- Bacteria, fungi, and mycoplasma detection.
Reporter Gene Cell Line Development
(Total 13-21 Weeks)
Service Workflow
I
Host Cell Detection (1-2 Weeks)
- Cell viability assessment.
- Cell count measurement.
- Bacteria and fungi detection.
- Mycoplasma detection.
II
Reporter Gene Expression Plasmid Construction (2-3 Weeks)
- Codon optimization.
- Whole gene synthesis.
- Construction of lentiviral expression plasmid.
III
Transient Transfection of Cells (1-2 Weeks)
- Transient transfection of host cells with the prepared reporter gene expression plasmid.
IV
Cell Pool Screening and Functional Activity Validation (1-2 Weeks)
- Positive cell selection.
- Measurement of cell pool expression levels.
- Drug-induced detection of signaling pathways.
V
Monoclonal Screening and Validation (7-10 Weeks)
- Single-cell plating and validation of monoclonal expression.
VI
Cell Line Quality Control (1-2 Weeks)
- Expansion cultivation of monoclonals, cryopreservation.
- Recovery viability assessment.
- Bacteria, fungi, and mycoplasma detection.
Customer provides:
Information on the target and host cells for the required cell line.
Final deliverables:
① Pool cells or monoclonal cells;
② Quality Inspection Report (COA) and Project Completion Report.
CUSABIO In-stock Cell Line Products: