What Are Histones?
DNA is like a magical, intricately coiled cable of life, but its wizardry lies in collaboration with histones, which magically compresses the 2-meter-long DNA within a tiny 6-micron cell, storing the secrets of genetic information.
1. What Are Histones?
Histone was first discovered by Albrecht Kossel in 1884 [1]. Histones are a family of alkaline proteins found in the nuclei of eukaryotic cells and are the major structural components of chromosomes. They are rich in lysine and arginine residues and thus positively charged, enabling their tight binding to the negatively charged DNA to form chromatin.
2. Structure and Classes of Histones
Histones are mainly divided into two classes: core histones and linker histones. Core histones include four members: H2A, H2B, H3, and H4. Linker histones include H1 or its homologue H5. In eukaryotes, the core histones are usually synthesized during DNA replication, reaching peak expression in the S phase.
All core histones show a strong conservation across species, from yeast to humans. They feature a C-terminal histone-fold domain (HFD) that is involved in histone octamer formation and an unstructured N-terminal tail that protrudes from the core particle and is subjected to various post-translational modifications (PTMs).
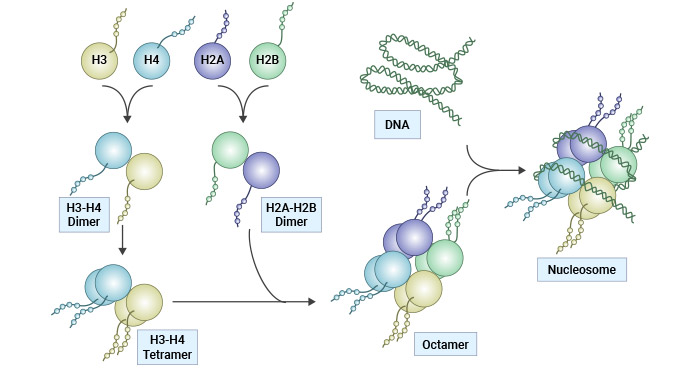
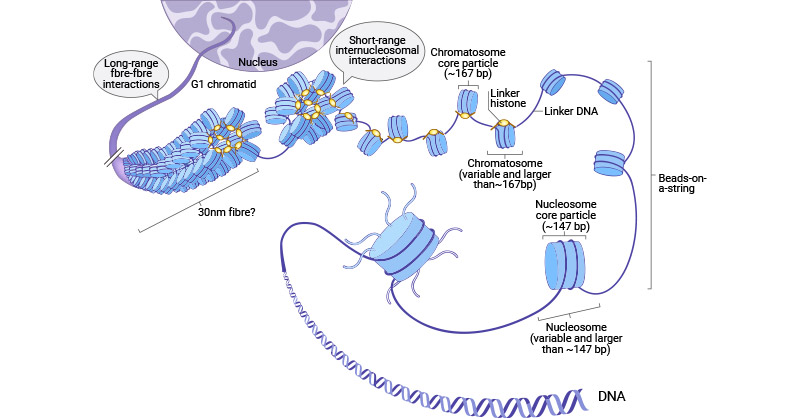
Within a nucleosome, the core histone consists of a (H3-H4)2 tetramer flanked by two H2A-H2B dimers, forming an octamer around which 147 bp of DNA is spooled twice [2]. The interaction between H1 and the nucleosome, along with additional DNA stretches at the entry/exit sites of the nucleosome, results in the formation of the beads-on-a-string chromosome and contributes to the establishment of higher-order chromatin structure [3-5]. Six nucleosomes, in conjunction with H1 histones, combine to form a solenoid structure, which is subsequently coiled around a scaffold, which is further coiled to assemble the chromosomal matrix.
Figure 1. Histone structure and nucleosome assembly
This picture is cited from: https://www.nature.com/articles/cddis2014337
Figure 2. Multiple levels of chromatin folding and histone structure
The picture is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5897046/
3. Histone Variants
Histone variants are variations or slightly different forms of histone proteins, particularly core histones. They share structural similarities with the core histones but have distinct properties and functions. They impart distinct structural and physical diversities to nucleosomes, either by wrapping more or less DNA or by changing nucleosome stability. Histone variants contribute to the complexity and versatility of chromatin structure and epigenetic genome regulation in eukaryotic cells.
The following is a list of human histone proteins and variants:
| Super family |
Family |
Subfamily |
Members |
| Linker Histones |
H1 |
H1F |
H1F0,
H1FNT,
H1FOO,
H1FX
|
| H1H1 |
HIST1H1A,
HIST1H1B,
HIST1H1C,
HIST1H1D,
HIST1H1E,
HIST1H1T
|
| Core Histones |
H2A |
H2AF |
H2AFB1,
H2AFB2/3,
H2AFJ,
H2AFV,
H2AFX,
H2AFY,
H2AFY2,
H2AFZ
|
| H2A1 |
HIST1H2AA,
HIST1H2AB/E,
HIST1H2AC,
HIST1H2AD,
HIST1H2AG, HIST1H2AI,
HIST1H2AJ,
HIST1H2AK, HIST1H2AL, HIST1H2AM
|
| H2A2 |
HIST2H2AA3,
HIST2H2AC
|
| H2B |
H2BF |
H2BFM, H2BC12L,
H2BFWT
|
| H2B1 |
HIST1H2BA,
HIST1H2BB,
HIST1H2BC/E/F/G/I,
HIST1H2BD,
HIST1H2BH,
HIST1H2BJ,
HIST1H2BK,
HIST1H2BL,
HIST1H2BM,
HIST1H2BN,
HIST1H2BO
|
| H2B2 |
HIST2H2BE
|
| H3 |
H3A1 |
HIST1H3A,
HIST1H3B, HIST1H3C, HIST1H3D, HIST1H3E, HIST1H3F, HIST1H3G, HIST1H3H, HIST1H3I, HIST1H3J
|
| H3A2 |
HIST2H3C |
| H3A3 |
HIST3H3
|
| H4 |
H41 |
HIST1H4A,
HIST1H4B, HIST1H4C, HIST1H4D, HIST1H4E, HIST1H4F, HIST1H4G, HIST1H4H, HIST1H4I, HIST1H4J, HIST1H4K, HIST1H4L
|
| H44 |
HIST4H4 |
The table information is cited from Wikipedia
4. Function of Histones
Histones were previously regarded as mere packaging material for nuclear DNA until their regulatory functions were uncovered in the early 1990s.
Critical factors determining histone function are the amino acid side chains found in proximity to histone N-terminal tails, which extend outward from the nucleosome core, providing access to enzymatic modification machinery capable of catalyzing various histone PTMs. Histone PTMs control the deposition and functions of histone proteins. The function of histones is mainly reflected in the following aspects:
4.1 DNA Packaging and Nucleosome Formation
Histones are responsible for packaging and condensing long strands of DNA into nucleosomes, which help further compact DNA and serve as a structural scaffold for a compact and organized chromatin. This compaction allows DNA to fit within the cell nucleus and prevents DNA from becoming tangled and protects it from damage.
4.2 Gene Regulation and and DNA Replication
Modifications such as methylation, acetylation, and phosphorylation of histones can modulate chromatin structure, thus affecting gene expression. For instance, histone methylation can repress gene expression, whereas acetylation is associated with active transcription.
4.3 Epigenetic Inheritance
Changes in histone modifications can be passed on to daughter cells during cell division, contributing to epigenetic inheritance. Epigenetic changes can influence gene expression patterns and cellular differentiation.
4.4 DNA Repair
Histones are involved in DNA repair processes. Specific histone variants, such as H2A.X, are rapidly phosphorylated at sites of DNA damage, marking those regions for repair.
4.5 Chromosome Segregation
During cell division, histones play a role in ensuring the accurate segregation of chromosomes into daughter cells. Modifications to histones can affect chromosome condensation and segregation.
4.6 Maintaining Genome Integrity
Histones help protect the integrity of the genome by preventing the exposure of DNA to damaging agents and enzymes.
4.7 Scaffolding for Protein Binding
Histones provide a scaffold for the binding of various proteins involved in transcription, DNA replication, and repair processes. These proteins can interact with specific histone modifications to carry out their functions.
5. Histone Post-modifications (PTMs)
Histones can be posttranslationally modified primarily in amino acid residues located on their N-terminal tails, usually in lysine, arginine, serine, threonine, and tyrosine residues. These modifications do not cause alterations to the DNA sequence but regulate chromatin structure, thus modulating gene expression.
Common histone modifications include methylation, acetylation, phosphorylation, SUMOylation, ubiquitination, and ADP-ribosylation. A single histone modification often does not function independently, but directs or inhibits another modification on the same histone, forming a cascade of modifications, which are recognized by a specific set of proteins and subsequently interpreted into a specific chromatin state to achieve regulation of a specific gene.
The crosstalk between different histone modifications serves as a signature or language, also known as the "histone code". Histone modifications can influence all DNA-associated functions, including gene transcription, chromatin assembly, DNA repair, and replication. The impact of these modifications hinges on both the specific functional group added and the precise site where it occurs.
References
[1] Kossel A, and Pringle H (1906). On protamines and histones [J]. H-S. Z. Physiol. Chem 49, 301–321.
[2] T.J. Richmond, C.A. Davey. The structure of DNA in the nucleosome core [J]. Nature, 423 (2003), pp. 145-150.
[3] P. Oudet, M. Gross-Bellard, P. Chambon. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit [J]. Cell, 4 (1975), pp. 281-300.
[4] Thomas JO: Histone H1: location and role [J]. Curr Opin Cell Biol. 1999, 11: 312-317. 10.1016/S0955-0674(99)80042-8.
[5] Hergeth SP, Schneider R. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle [J]. EMBO Rep. 2015;16(11):1439–1453.