mRNA Vaccine Research Solutions
mRNA vaccines represent a groundbreaking immunization technology harnessing the biological properties of messenger RNA (mRNA) to induce immune responses. These vaccines consist of a segment encoding a specific antigenic protein's mRNA sequence. Typically, the antigenic protein is a crucial surface protein of a pathogen, such as the spike protein of coronaviruses or the hemagglutinin protein of influenza viruses. Once introduced into the body, the mRNA guides cells to produce these antigenic proteins, triggering an immune response and effectively preventing diseases. Compared to traditional vaccines, mRNA vaccines offer advantages in rapid development, a relatively straightforward and swift production process, and exceptional safety and efficacy.
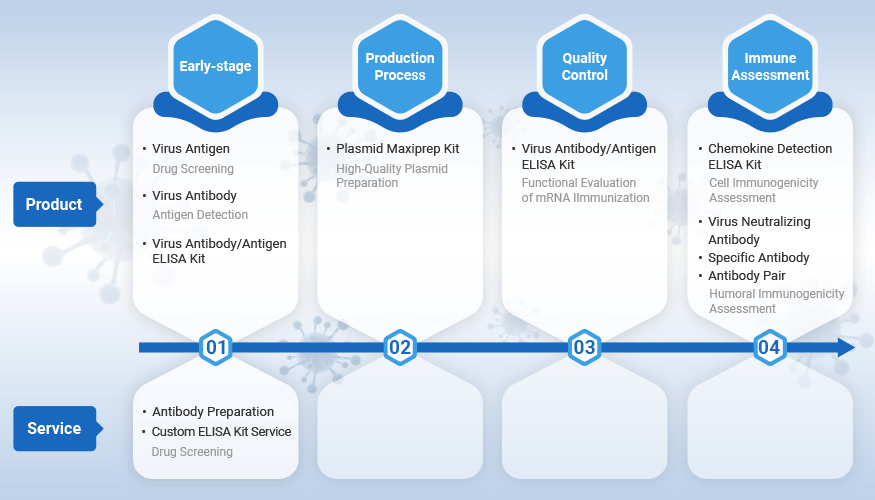
With years of expertise in protein, antibody, and ELISA kit development, CUSABIO possesses an extensive range of research reagents for viral studies and provides high-quality technical services. We are dedicated to supporting various stages of mRNA vaccine development, including early-stage research, production, quality control, and immune potency testing. Our commitment is to advance the progress of mRNA vaccine research.
Early-Stage Research and Development
During the early stages of research and development, CUSABIO offers a diverse range of products and customized services for virus antigen recombinant proteins, antibody detection, ELISA kits, and more. These resources are designed for antigen detection, drug screening, and vaccine development purpose.
Virus Antigen Products
Virus recombinant proteins are essential materials for the production of mRNA vaccines, and they find applications in early-stage drug screening and vaccine development.
CUSABIO has developed over 1000 types of virus antigen recombinant proteins, spanning a wide range of mainstream viruses, including influenza diseases, rabies viruses, coronaviruses, hepatitis viruses, and more.
More
Recombinant Protein Expression Services
CUSABIO possesses extensive experience and technical expertise in recombinant protein expression. We offer a comprehensive range of five major recombinant protein expression systems, including prokaryotic, eukaryotic, and cell-free systems, to meet diverse requirements for expressing different virus antigens. Our services cover protein purification, quality detection, and control, ensuring the quality and purity of recombinant proteins. This comprehensive approach serves as a crucial antigen source for the preparation of mRNA vaccines.
More
Virus Antibody Products
CUSABIO offers a variety of detection antibodies designed for the identification and assessment of antigenic proteins within mRNA vaccines. Examples include SARS-CoV-2 antibodies and rabies virus M protein antibodies. These antibodies are suitable for immunological detection methods such as ELISA and Western blot, providing a means to evaluate the immunogenicity of vaccines.
More
Custom Antibody Services
With extensive experience and professional technical expertise, CUSABIO offers comprehensive Custom Antibody Services. Tailored to the nature of the antigen and specific immunological requirements, our one-stop service includes antigen design, immunogen preparation, hybridoma cell screening, and antibody production. This service is designed to assist researchers in mRNA vaccine development in quickly obtaining high-quality, specific antibodies targeting particular antigens.
More
Virus Antibody/Antigen ELISA Kit Products
During the early stages of research and development, virus antigen assay kits can be employed to detect and identify antigenic proteins within mRNA vaccines. This facilitates the evaluation of vaccine immunogenicity, allowing for an understanding of the induced immune response levels and the subsequent determination of vaccine effectiveness and safety.
CUSABIO offers a variety of virus antigen ELISA kits characterized by high sensitivity and specificity. These kits provide valuable tools for assessing the immunogenicity of mRNA vaccines.
More
Custom ELISA Kit Services
CUSABIO, equipped with proprietary primer/gene synthesis, protein expression, antibody preparation, and assay kit development platforms, offers comprehensive one-stop services from immunogen to ELISA kit production. With a high degree of flexibility, our services allow for the selection of different target antigens, detection modes, and labeled antibodies based on specific requirements, meeting the unique needs of various vaccine development projects. Our rigorous adherence to eight major quality control standards ensures the production of ELISA kits with high sensitivity, specificity, and stability.
More
Production Process
In the manufacturing of mRNA vaccines, plasmids serve as carriers, into which foreign genes are inserted, followed by transcription and translation processes to generate the corresponding mRNA. CUSABIO provides a Plasmid DNA Purification Maxiprep Kit, enabling you to rapidly and efficiently extract and purify plasmid DNA, thereby supplying the necessary raw material for mRNA vaccine production. The extracted and purified plasmids can be used in subsequent experiments, such as enzyme digestion and transfection, to generate mRNA with specific sequences.
Quality Control
Once mRNA is translated into functional antigenic proteins, it induces the production of antigen-specific immune responses in the body, forming immunological memory. The second edition draft of the United States Pharmacopeia's mRNA Vaccine Quality Analysis (Analytical Procedures for mRNA Vaccine Quality) mentions that a functional binding assay (cell-based assay) for the expressed protein in transfected cells can be employed to demonstrate the efficacy of mRNA-LNP (confirm the integrity of mRNA molecules and demonstrate critical epitopes of the expressed protein).
The product-specific assessment of proteins encoded by RNA can be analyzed through cell-based protein expression methods, such as the ELISA approach. ELISA methods enable the detection of protein expression at the cellular level and the evaluation of the binding functionality of the expressed protein.
Utilizing ELISA methods for assessing the functionality of antigenic proteins encoded in mRNA vaccines during the quality control phase is a crucial means to ensure vaccine safety and efficacy. Through this detection method, the performance and quality of the vaccine can be evaluated, providing robust support for subsequent clinical trials and production. CUSABIO offers various common virus antigen ELISA kits with high sensitivity and specificity, suitable for performance and quality assessment during the quality control phase of vaccine development.
Virus Antibody/Antigen ELISA Kit Products
Immune Potency Testing
During the immune potency testing phase of mRNA vaccine development, multiple indicators are examined, including antibody levels, cellular immune responses, secretion of cytokines and chemokines, specific antibody production, immune memory, and cross-reactivity. By comprehensively analyzing these indicators, it is possible to understand the characteristics of the immune response and protective effects induced by the vaccine, providing crucial information for vaccine development and improvement.
● Cytokine and Chemokine Detection
Cytokines are crucial regulatory factors in the immune system, playing a key role in the immune response process. Detecting immune-related cytokines and chemokines produced by cells after vaccine administration helps to understand the characteristics and mechanisms of the immune response induced by the vaccine.
Cytokine Detection ELISA Kits
CUSABIO offers a range of Cytokine ELISA kits covering 180+ cytokine targets. With high sensitivity, precision, and stability, these kits effectively meet diverse research needs related to cytokines, providing optimal support for cytokine-related scientific investigations.
More
Chemokine Detection ELISA Kits
As a category of cell factors with crucial physiological functions, CUSABIO offers Chemokine ELISA kits that virtually encompass all types of chemokines and their receptors. All products in this line exhibit high sensitivity, precision, and stability, providing reliable tools for the detection of chemokines in various research applications.
More
● Specific Antibody Detection
By detecting the specific antibodies produced in the body after vaccination, it is possible to understand the type and specificity of the immune response induced by the vaccine. Common detection methods include ELISA, immunoblotting, and immunoprecipitation.
Virus Antibody ELISA Kits
Virus-Specific Antibodies
● Virus Neutralizing Antibody Detection
Neutralizing antibody detection involves assessing the level of specific antibodies produced in the body after vaccination to evaluate the strength and immunogenicity of the immune response induced by the vaccine. Common detection methods include enzyme-linked immunosorbent assay (ELISA) and neutralizing antibody assays.
Virus Neutralizing Antibody Detection ELISA Kits
CUSABIO offers Virus Neutralizing Antibody Detection ELISA kits, such as the SARS-CoV-2 Neutralizing Antibody ELISA Kit. Customization services for Virus Neutralizing Antibody Detection ELISA kits are also available based on customer requirements.
More
Virus Neutralizing Antibodies
Neutralizing antibodies can bind to antigens on the surface of a virus, directly preventing the virus from attaching to target cell receptors and thus blocking viral entry into host cells. Neutralizing antibodies are typically generated in animals infected with the pathogen, and one of the prerequisites for successfully preparing neutralizing antibodies is the production of highly effective and specific antisera. CUSABIO employs in-house adjuvants and has established an efficient antibody screening mechanism, offering customization services for virus neutralizing antibodies based on customer requirements.







