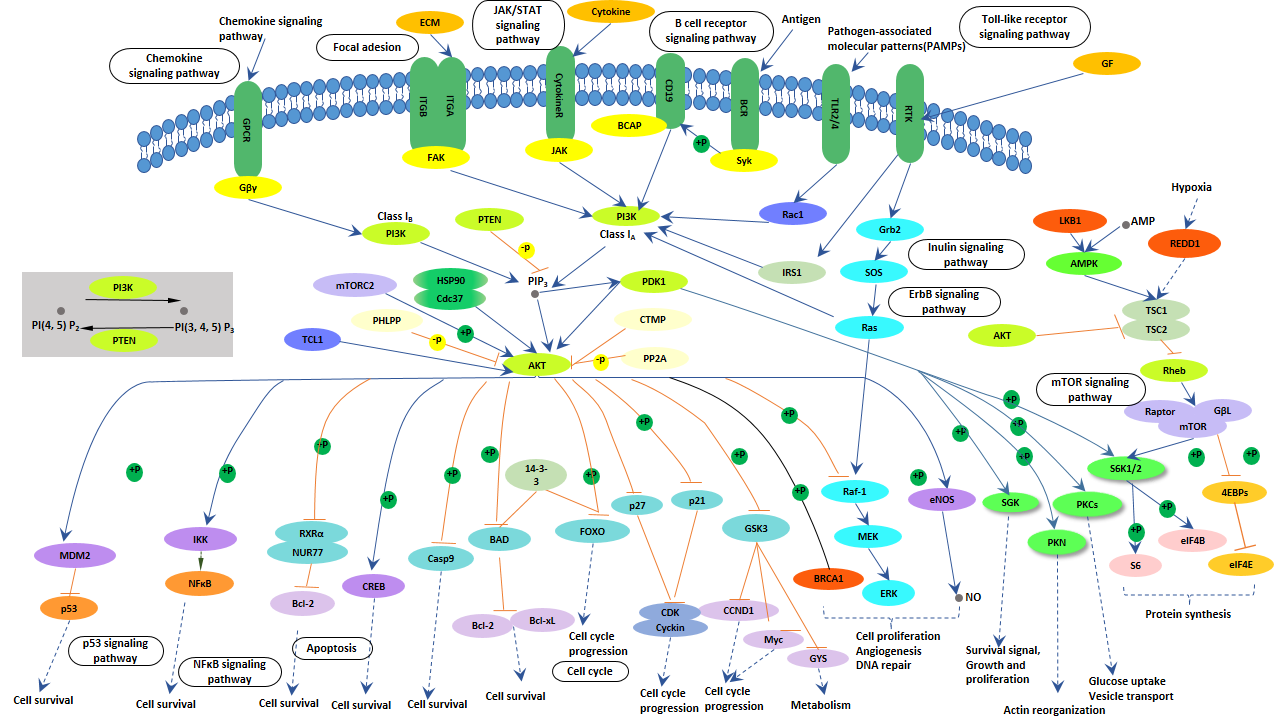
What is PI3K-Akt Signaling Pathway?
The phosphatidylinositol 3' –kinase (PI3K)-Akt signaling pathway is an intracellular signaling pathway important in regulating the cell cycle and is activated by many types of cellular stimuli or toxic insults.
The Function of PI3K-Akt signaling pathway
It regulates fundamental cellular functions such as transcription, translation, proliferation, growth, and survival in response to extracellular signals. This is mediated through serine and/or threonine phosphorylation of a range of downstream substrates.
The Processes of PI3K-Akt Signaling Pathway
As shown in following picture, activation of growth factor receptor protein tyrosine kinases including epidermal growth factor receptor (EGFR) by external growth factors results in auto-phosphorylation on tyrosine residues and subsequent events to activate these intracellular pathways.
PI3K is recruited to the membrane by directly binding to phosphotyrosine consensus residues of growth factor receptors or adaptors through one or both SH2 domains in the adaptor subunit. This leads to allosteric activation of the catalytic subunit. Activation results in production of the second messenger phosphatidylinositol-3, 4, 5-trisphosphate (PIP3).
The lipid product of PI3K, PIP3, recruits a subset of signaling proteins with pleckstrin homology (PH) domains to the membrane, including PDK1 and Akt. PTEN, is a PI-3, 4, 5-P3 phosphatase, which negatively regulates the PI3K/Akt pathway.
Once activated, Akt mediates the activation and inhibition of several targets, resulting in cellular survival, growth and proliferation through various mechanisms. In the mechanism of PI3K-Akt signaling pathway, the key molecules involved in this signaling pathway are receptor tyrosine kinase (RTKs), phosphatidylinositol 3-kinase (PI3K), phosphatidylinositol-4,5-bisphosphate (PIP2), phosphatidylinositol-3,4,5-bisphosphate (PIP3) and AKT/protein kinase B.
The PI3K-Akt Signaling Pathway and Cancer
Most genetic alterations associated with tumor phenotype are representative of a finite succession of physiologic disturbances, which, collectively, render the cell to become malignant. Alterations of the PI3K-Akt signaling pathway have been reported in numerous human cancers. The PI3K-Akt signaling pathway plays an important role in the characteristic process of cancer. For example, Akt overexpression or activation may lead to an increased response to ambient levels of growth factors. Sustained activation of Akt makes tumor cells insensitive to anti-proliferative signals by inducing nuclear entry of Mdm2, which leads to inhibition of p53 regulated processes and by inducing cytoplasmic localization of p21Cip/Waf1 and p27Kip, which promotes proliferation. Akt activation also suppresses apoptosis of cancer cells by inactivating pro-apoptotic factors Bad and pro-caspase-9, but by activating IKK that provokes the transcription of NF-κB regulated antiapoptotic genes. Besides, the PI3K-Akt pathway also promotes tumor angiogenesis through eNOS activation and contributes to invasiveness by inhibiting anoikis and stimulating MMP secretion.
There are numerous aberrant mutations of the PI3K-Akt pathway in human cancers, including loss of the lipid phosphatases PTEN and INPP4B, as well as mutation and amplification of the genes encoding the PI3K catalytic subunits p110α (PIK3CA) and p110β (PIK3CB), and so on. In recent clinic cancer treatment, several drugs targeting the PI3K-Akt pathway have been developed and are currently in clinical trials in different phases of clinical development, such as PI3K Inhibitors, Isoform-Specific PI3K Inhibitors, Dual PI3K/mTOR Inhibitors. However, these drugs are just observed early signals of clinical activity. A better understanding of this essential crossroad between PI3K-Akt signaling and cancer is conducive to fully exploit the potential benefits of these new drugs.
The PI3K-Akt Signaling Pathway and Diabetes
Under normal conditions, insulin is immediately secreted after a meal. The released insulin binds to and activates IRS-1/2 (insulin receptor substrate-1/2), initiating the PI3K-Akt signaling pathway. Insulin-mediated Akt pathway accelerates glucose utilization, reduces gluconeogenesis of liver and muscle, increases body lipid deposition, thereby reducing free fatty acid (FFA) circulation in adipose tissue, increases pancreatic insulin secretion, and regulates lipid and glucose metabolism balance, reduce brain appetite. However, in the case of chronic energy excessive conditions, such as obesity, lipid accumulation is saturated, resulting in increased lipolysis of adipose tissue, leading to excessive FFAs. Lipid ectopic accumulation of skeletal muscle leads to reduced glucose transport and glycogen synthesis. Excess FAAs also disrupts β-cell function and insulin secretion. In the liver, excess FAAs suppress extrahepatic insulin signal transduction and ectopic accumulation of lipids, leading to an increase in HGP (hepatic glucose production) and DNL (de novo lipogenesis). In the brain, excessive FFAs cause glucose and lipid metabolism disorders. All these eventually impairs the PI3K-Akt signal, inducing insulin resistance. Insulin resistance further exacerbates the PI3K-AKT signal, forming a vicious circle that leads to obesity and Type II diabetes. As the PI3K-Akt pathway is closely related to metabolism, regulation of PI3K-Akt signaling pathway and its downstream molecules is a potential therapeutic target for the treatment of obesity and type 2 diabetes.





