Q: How to choose a membrane protein technology platform?
A: Different membrane protein technology platforms have their own advantages and disadvantages. The selection should be based on the specific protein expression needs and application scenarios. You can consult with technical personnel from CUSABIO for specific guidance. Below is a comparison of different platforms:
| Technology Platforms |
Virus Like Particles (VLP) Platform |
Detergent Micelle Platform |
Nanodisc Platform |
| Coating for ELISA |
Direct coating |
Direct coating |
Direct coating |
| Activity |
Great |
Good |
Good |
| Stability |
Most stable |
Relatively stable |
Relatively stable |
| Purity |
Mixture |
Relatively pure (only target protein) |
Mixture |
| SPR/BLI |
Detectable |
Detectable (solution adjustment required) |
Detectable |
| Factors to consider |
Total protein concentration; relatively short preparation cycle |
Relatively long preparation cycle |
Relatively long preparation cycle |
Q1: Are any viruses or viral vectors used in the preparation of membrane proteins with the VLP platform?
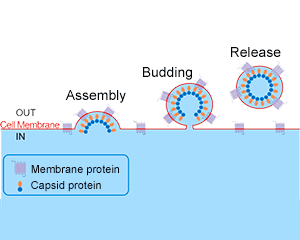
A: Mammalian enveloped VLPs are enveloped virus-like particles, which are self-assembled by one or several structural proteins of the virus into a virus-free genome that cannot replicate and have no infectivity, but are similar in shape and structure to complete viruses and do not contain viral nucleic acid substances. It’s safe. Now many vaccines will also choose this technology, which shows that it is safe.
Mammalian enveloped VLPs are co-transfected with two expression plasmids (expressing transmembrane protein and capsid protein respectively) without using any virus and viral vector, and the transfection reagents are used for transient transfection.
Q2: How many liposomes and membrane skeleton proteins combinations are selected for Nanodisc platform, and do you optimize the combination for each project?
A: In the early stage of platform development, a variety of membrane skeleton proteins and phospholipid molecules were tried, and orthogonal combinations were attempted, and finally a pair of optimal membrane skeleton proteins and phospholipid molecules was screened out. At present, the platform is mature and we use this same combination for different projects.
Q3: What is the coating concentration of VLPs and how much is used?
A: Considering that the total concentration of VLPs is measured, the recommended coating concentration is 2-10ug/ml, and the amount used for one well is 0.2-1ug.
Q4: What is the concentration of your VLPs?
A: The Bradford assay method is used for the concentration determination. The specific concentration of each item and each batch is different. Please consult the concentration information of the corresponding batch during pre-sales inquiry.
Q5: When VLPs is used as an antigen to immunize antibodies, does VLPs have any effect on the quality of antibodies, and the effect of different adjuvants?
A: At present, we have tested, without adjuvant, adding rapid adjuvant (without emulsification) and conventional Freund's adjuvant to evaluate the impact on immunity. From the final effect, it is recommended to use rapid adjuvant.
Q6: Have you successfully made a candidate antibody drug molecule using the protein developed from this platform?
A: There are many, but it involves customers projects, it is inconvenient to inform.
Q7: Is VLPs output production stable and what is the approximate level?
A: Relatively stable, and the specifics are also related to each project (the specific data is temporarily confidential).
Q8: If the customer's antibody does not recognize the protein, whether it belongs to the category of risk-free protection?
A: Considering the difference of different positive antibodies, we will generally produce a positive antibody by ourselves. If both of the customer's antibody and our produced positive antibody don’t bind, it belongs to the risk-free category (no charge for inactivity).
Q9: Do you provide empty VLPS as a control? In your quality control data, what is the measured value of the empty VLPS negative control?
A: We can provide empty VLPs as control for free, the negative control value is 0.1 or lower.
Q10: Why can VLPs form stable membranes?
A: The envelope of VLPs is derived from the cell membrane and itself is a stable membrane.
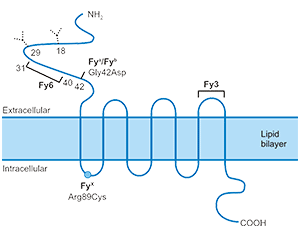
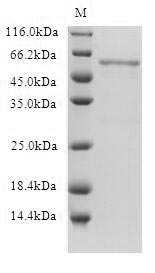
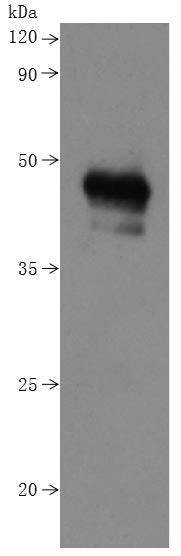
Q11: Why does the WB image of Claudin18.2 have three bands?
A: The three bands coorespond to monomer, dimer and trimer respectively.
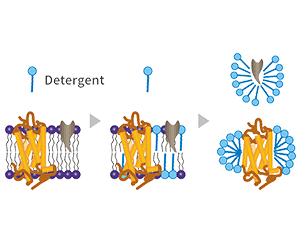
Q12: Can you please share any tips regrading safe detergents and its concentrations that I can use to extract the recombinant proteins from the VLP particles?
A: We are sorry to tell you that VLPs and target protein cannot be separated, because VLPs protein is an enveloped virus-like particles, and the target protein is displayed on the surface. The target protein can be close to the natural conformation in VLPs. So the VLPs protein also cannot be accurately quantified. But we provide the VLPs control protein under the same preparation conditions for free to the customer to deduct the influence of the host membrane protein on the envelope.
Q13: Regarding the Virus-Like Particles (VLPs) Mammalian cell expression system , could you please let me know in which buffer the protein is provided ? Also as it is membrane protein, is the protein supplied in liposome, or will it be solubilized?
A: The standard buffer that we normally use is PBS, pH 7.4, 6% trehalose. We can etither provide it in liquid form or lyophilized form, please communicate with us in advance if you have any special requirement for the buffer or the shipping format.
Liquid form: It needs to be shipped with dry ice and we need to charge extra fees for dry ice and dry ice box.
Lyophilized form: It could be shipped with normal bule ice packs and no extra charges. However, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
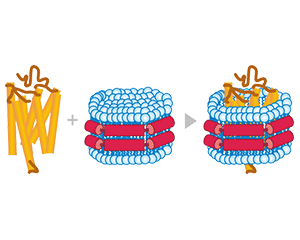
Transmembrane proteins are displayed on the nanoparticle envelope in their native state.
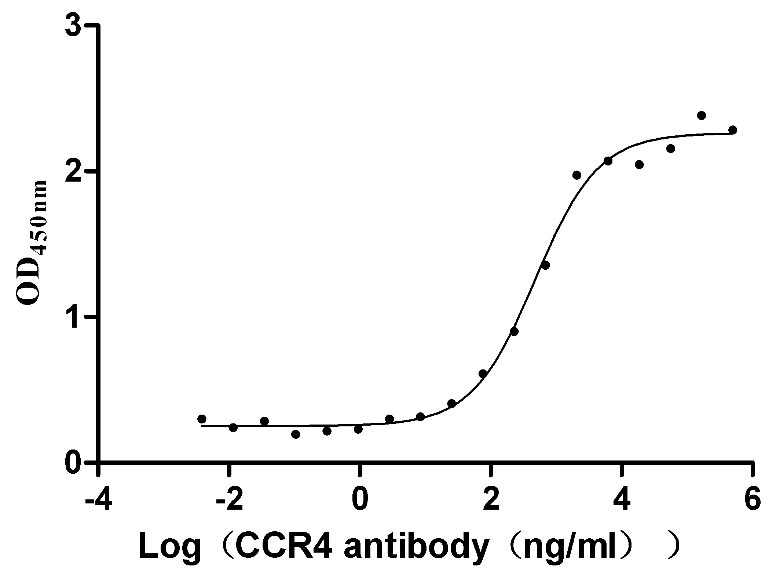
Q14: Can we think the the structure of #CSB-MP3838 (Virus-Like Particles (VLPs) isotype control) and #CSB-MP004843HU are identical except for CCR4?
A: Yes, the structure are identical except for CCR4.
Q15: For immunization and antibody screening, which platform should I choose for Claudin18.2?
Our Claudin18.2 has been developed with VLP platform. Both of VLP version and the detergent version are suitable for immunization and screening. However, the VLP version may be preferred for improved immunogenicity. For accurate affinity measurements, maybe the detergent version is more suitable.
Q16: Why do you recommend to express the full-length transmembrane protein? After all, antibodies and antigens only bind on the extracellular domain.
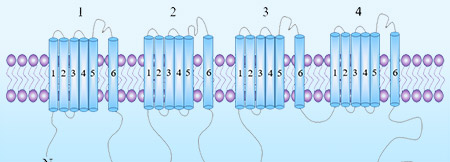
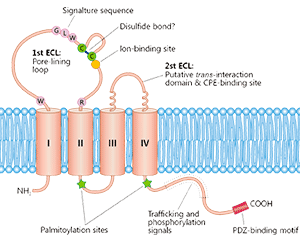
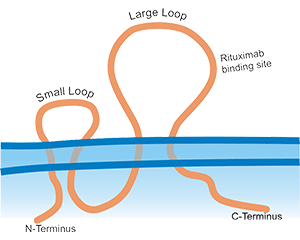
Multi-pass transmembrane proteins span the cell membrane multiple times forming multiple extracellular domains. For example, Claudin18.2 and CD20 have two extracellular loops (ECLs) with each ECL having specific functions and interactions with each other. Full length can ensure that the protein conformation is biologically relevant while enabling the ECL to be completely exposed for improved screening of ideal antibodies. According to our experience, the full length is active and relevant compared to isolated extracellular domain. It is not the case that CUSABIO always emphasizes full length proteins, but drug discovery R&D work needs the full-length multi-pass transmembrane proteins. Indeed, when compared, the activity of only the ECL region is worse than that of the full-length protein. CUSABIO is committed to providing the high quality and relevant products that meet customers' needs.
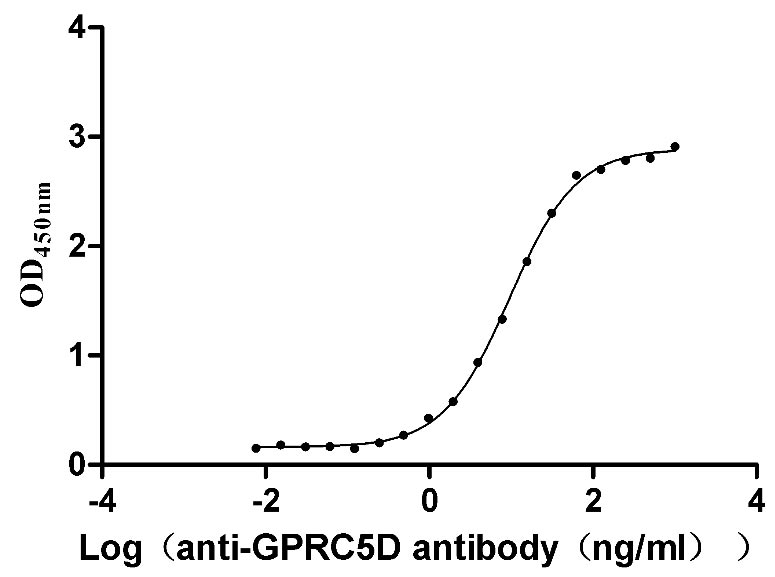
17: How do you confirm that Claudin18.2-VLP contains the target protein and how do you evaluate its purity? In the EM image on your product page, how do you tell whether the protein is embedded on the VLP particle?
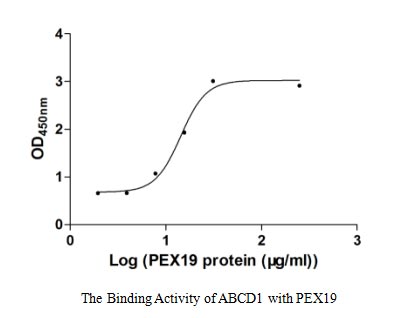
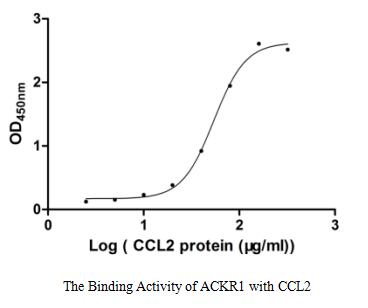
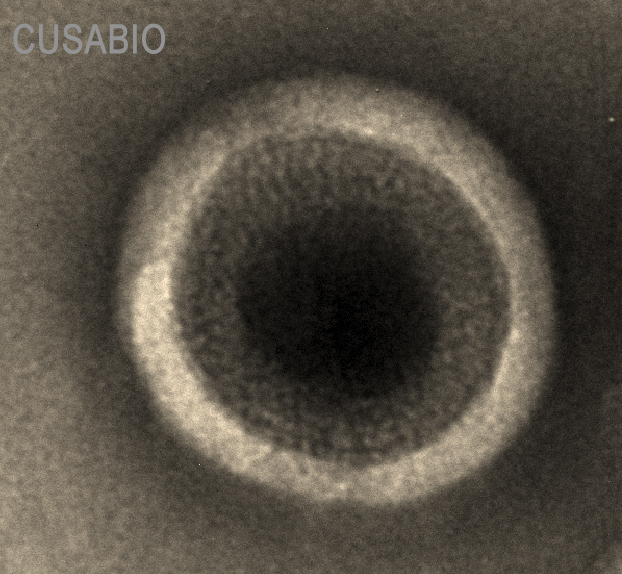
Claudin18.2-VLP is tested and verified by anti-Claudin18.2 specific antibodies. The existence is evaluated by WB/SEM. The conventional negative dye EM cannot see whether Claudin18.2 is on the VLP due to its low resolution. Binding activity with anti-Claudin18.2 specific antibodies can confirm the presence of Claudin18.2 on the particles.
Q18: Are you able to provide VLP-membrane proteins in lyophilized form? Could it be emulsified, and are there any requirements for the type of adjuvant? What are the precautions for using VLP formulated proteins for immunization?
After our continuous technical optimization, we can now provide VLP-membrane proteins in lyophilized form, which can be transported by normal blue ice packs. VLP immunization mainly requires attention to the choice of adjuvant dose, because VLP itself can enhance immunogenicity, which is not the case for conventional protein products.
Q19:How long does it take for the VLP platform to develop successfully?
A: Generally, it will take 1.5 to 2.5 months, including the activity test. If customers have positive antibodies, they can send us to test activity.

-SDS.jpg)
-WB.jpg)
-TEM1.jpg)
-AC1.jpg)
-AC1.jpg)
-AC1.jpg)
-AC1.jpg)