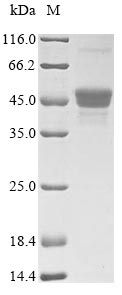
Recombinant Candida albicans pH-regulated antigen PRA1 is produced using a yeast expression system, covering amino acids 16-299 of the mature protein. The full-length protein includes an N-terminal 6xHis tag to simplify purification and detection. SDS-PAGE analysis confirms the product achieves greater than 90% purity, which appears to ensure reliable performance in research applications. Endotoxin levels are kept low to support consistent experimental outcomes.
PRA1, a pH-regulated antigen from Candida albicans, seems to play a significant role in the organism's virulence and adaptability. The protein is involved in how this fungal pathogen responds to environmental pH changes, affecting its ability to survive and persist in different conditions. Understanding PRA1's function may be crucial for research into fungal pathogenesis and host-pathogen interactions. This makes it an important target in studies of Candida infections, though the exact mechanisms remain an active area of investigation.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
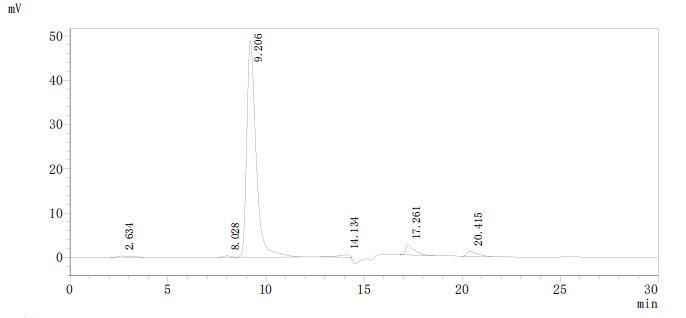
Based on the provided information, the recombinant Candida albicans PRA1 protein is expressed in a Yeast expression system, which is phylogenetically similar to Candida albicans (both are fungi), increasing the likelihood of proper folding and post-translational modifications. The high purity (>90% by SDS-PAGE and SEC-HPLC) suggests a homogeneous preparation, and SEC-HPLC results indicate the protein is likely monodisperse, which often correlates with correct folding. However, since activity is unverified, the protein cannot be guaranteed to be correctly folded or bioactive without functional validation (e.g., binding assays or immunological activity tests). While the expression system supports a high probability of correct folding, experimental confirmation is essential.
1. Antigen-Antibody Interaction Studies
This recombinant PRA1 protein can function as an antigen for developing and characterizing antibodies against Candida albicans. The N-terminal 6xHis tag allows for straightforward immobilization on nickel-coated surfaces during ELISA-based antibody screening and binding affinity measurements. With >90% purity, the protein likely provides reliable and reproducible results in antibody development workflows. Researchers might use this protein to evaluate cross-reactivity patterns and the specificity of anti-Candida antibodies in controlled in vitro assays. However, if the protein is misfolded, antibodies generated may not recognize conformational epitopes of the native PRA1 protein in its physiological context, so validation against native PRA1 is recommended.
2. Protein-Protein Interaction Analysis
The 6xHis tag makes pull-down experiments technically feasible for identifying potential binding partners of PRA1 from Candida albicans cell lysates or other protein mixtures. The recombinant protein can be immobilized on nickel affinity resins to capture interacting proteins. However, if PRA1 is misfolded, it may not interact physiologically with true binding partners, leading to non-specific or false interactions. The high purity level should minimize background binding, but results should be interpreted cautiously until folding is validated through functional assays. If correctly folded, this application is valuable; otherwise, it may yield misleading data.
3. Biochemical Characterization and Stability Studies
The purified recombinant PRA1 protein allows for comprehensive biochemical analysis, including determination of molecular weight, isoelectric point, and thermal stability profiles. Researchers can investigate how the protein behaves under various pH conditions—particularly relevant given its designation as a pH-regulated antigen. The yeast expression system likely ensures proper eukaryotic folding, making it suitable for studying native-like biochemical properties. These studies can directly assess folding status (e.g., via circular dichroism or SEC-HPLC) and are valuable even if the protein is misfolded, as they characterize the recombinant product. The description is correct.
4. Immunological Research Applications
This recombinant PRA1 can be used to study immune responses in cell culture models by exposing immune cells to the purified antigen and measuring cytokine production or cellular activation markers. The protein serves as a standardized antigen for investigating host-pathogen interactions. However, if misfolded, conformational epitopes may be altered, potentially leading to non-physiological immune responses. The high purity supports reproducibility, but researchers should validate findings using native PRA1 or in vivo models to ensure relevance. The description is generally correct but requires caution for interpretation.
Final Recommendation & Action Plan
Given the high probability of correct folding due to the homologous yeast expression system and high purity, but without activity verification, it is recommended to first perform biochemical and functional validation to confirm protein folding and bioactivity. Prioritize applications like biochemical characterization and antibody development, which are less dependent on native conformation, while postponing protein-protein interaction studies until folding is confirmed. Use techniques such as circular dichroism for secondary structure analysis and functional assays (e.g., binding to known partners or antibodies) to validate activity. If the protein is active, it can be reliably used for all described applications; if not, focus on non-functional uses like antigen production or stability studies. Always include controls with native PRA1 when possible to ensure physiological relevance.