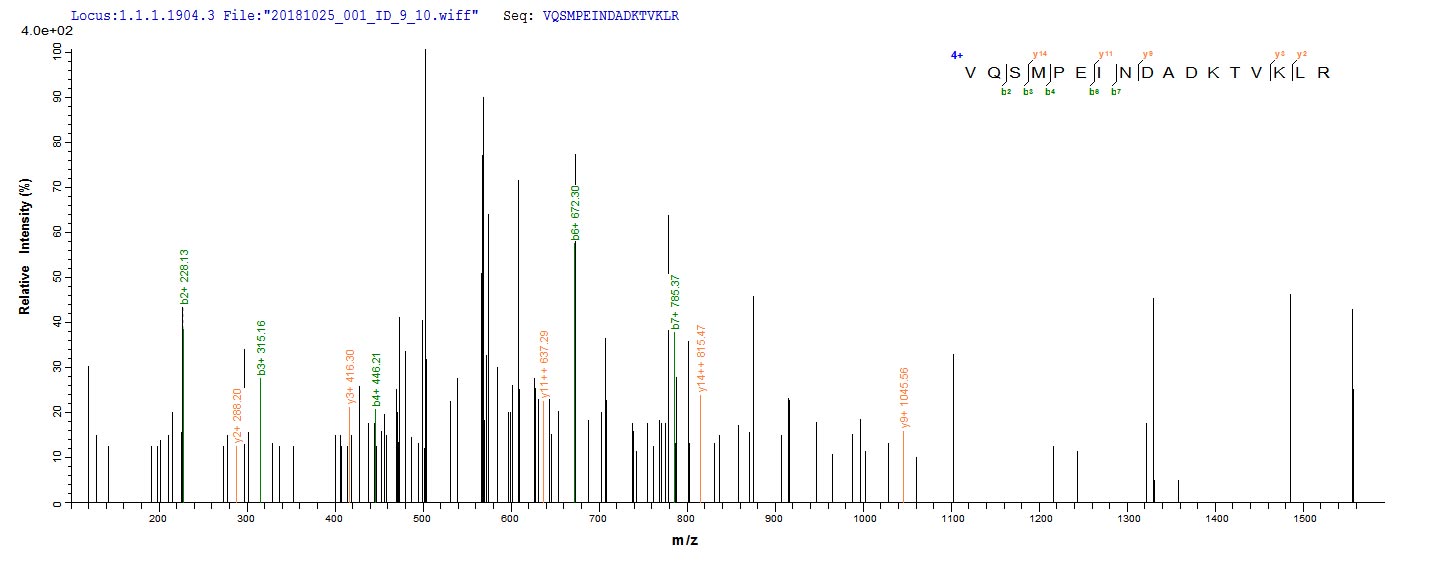
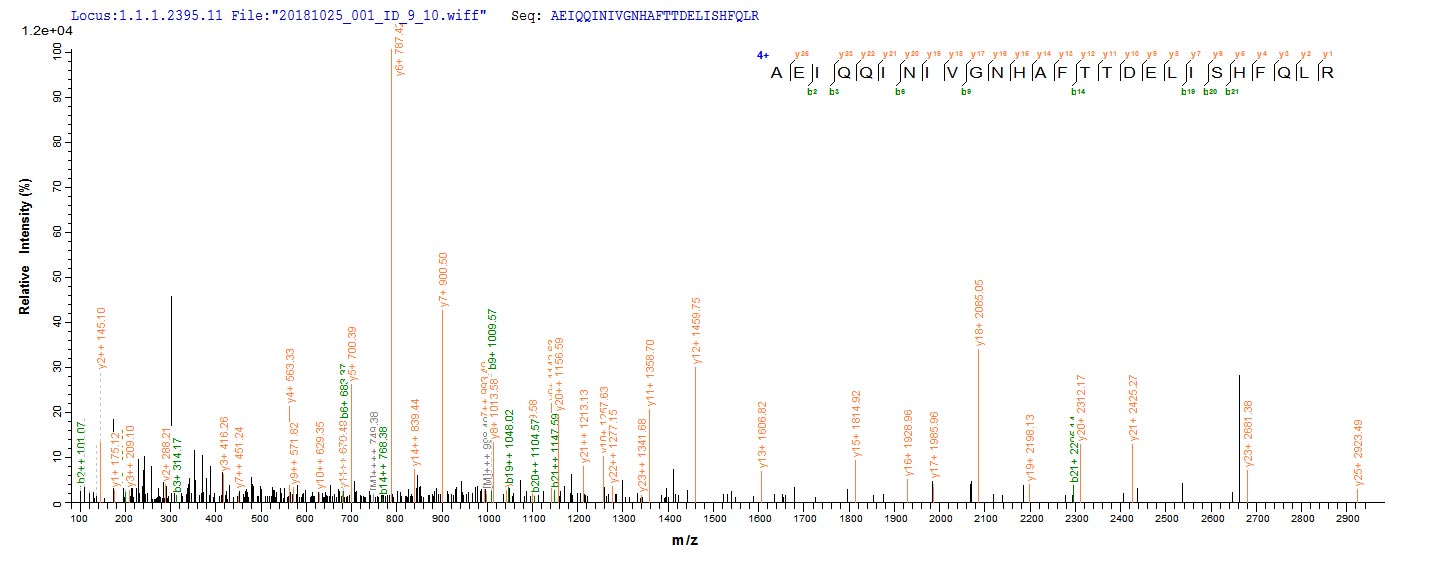
The recombinant Escherichia coli (strain K12) bamA was expressed with the amino acid range of 175-424. The theoretical molecular weight of the bamA protein is 36.0 kDa. This bamA recombinant protein is manufactured in e.coli. Fusion of the N-terminal 10xHis tag and C-terminal Myc tag into the bamA encoding gene fragment was conducted, allowing for easier detection and purification of the bamA protein in subsequent stages.
Escherichia coli Outer membrane protein assembly factor BamA is a central component of the β-barrel assembly machinery (BAM) complex, crucial for the proper folding and insertion of outer membrane proteins (OMPs). Research on BamA primarily explores its role in outer membrane biogenesis, focusing on understanding its structure, mechanism, and interactions with substrates. Investigations aim to elucidate the intricate processes of protein folding and insertion, providing insights into bacterial outer membrane integrity and potential targets for antimicrobial drug development, crucial for combating bacterial infections and addressing antibiotic resistance.