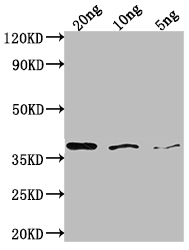
The production of the bamA polyclonal antibody is carried out through a carefully orchestrated procedure. It begins with the repeated immunization of a rabbit using recombinant Escherichia coli Outer membrane protein assembly factor bamA, partial (175-424AA) until a sufficient antibody titer is achieved. Subsequently, the rabbit's blood is collected, and the antibodies are purified from the serum utilizing protein A/G. The functionality of the resulting bamA antibody is rigorously assessed in ELISA and WB applications, conclusively demonstrating its specific binding to the Escherichia coli bamA protein.
E.coli bamA is the central constituent of the β-barrel assembly machinery (BAM) complex and plays a crucial part in outer membrane protein (OMP) assembly. It is characterized by a discontinuous β-barrel structure with compromised interactions between the β1-β16 strands, featuring only two connecting hydrogen bonds, along with a disordered C-terminus.