Pharmacokinetic (PK) assays play a pivotal role in pharmacology, offering essential insights into how drugs are absorbed, distributed, metabolized, and excreted in the body. They provide crucial understanding regarding drug behavior from circulation to its final form after metabolism. As the focus on antibody-drug conjugates (ADCs) intensifies, the significance of PK assay results in shaping ADC clinical trial designs becomes even more apparent. This year, the ADC field continues to advance at a rapid pace, particularly with the upgraded DXD ADCs making remarkable breakthroughs in innovative anti-tumor approaches!
DXD-the Most Promising Payload for Next Generation ADCs
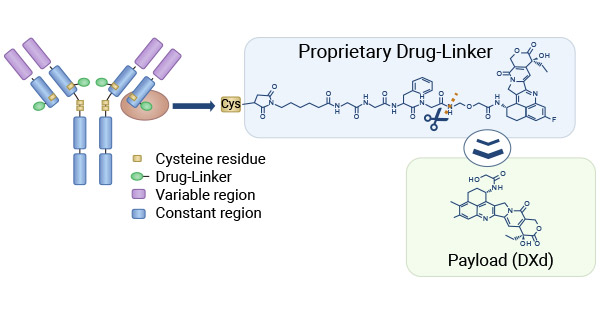
DXD (Exatecan derivative for ADC) is a novel DNA topoisomerase I inhibitor, which belongs to the class of compounds known as camptothecin (CPT). As potent ADC payloads, DNA topoisomerase I inhibitors exhibit great promise by inducing DNA damage, apoptosis through single-stranded DNA cleavage, and inhibition of the topoisomerase repair mechanism. The new generation of DXD-ADC drug has desirable characteristics for ADC including i) a highly potent payload (DXD) with a short systemic half-life; ii) a cleavable linker designed to be tumor selective which is stable in circulation; iii) an average drug-to-antibody ratio (DAR) up to 8 being optimized for each target, iv) particularly, DXD with more potent inhibitory activity of Topo I than SN-38 [1-4].
These features enable the wide therapeutic window of the ADC with high anti-tumor potency and less systemic toxicity. Based on the new DXD ADC technology, there are several new ADCs in clinical development: DXd ADC (TDxd/DS-8201/Enhertu), Dato-DXd (DS-1062), HER3-DXd (U3-1402/Patritumab deruxtecan), DS-7300, DS-6000 and DS-3939, targeting HER2, Trop2, HER3, B7-H3, CDH6, and MUC1, respectively [1-4].
Figure. 1 DXD-the most promising payload for next-generation ADCs [1]
Pharmacokinetics (PK) of ADCs
The pharmacokinetics analysis of Antibody-Drug Conjugates (ADCs) exhibit similarities to those of conventional monoclonal antibody (mAb), but they are further influenced by the characteristics of the cytotoxic drug (small molecule drug). This interplay can enhance the diversity observed within ADCs. In assessing the pharmacokinetic behavior of ADCs, researchers commonly analyze various parameters, such as total antibody, ADC or conjugated mAb, and unconjugated drug in blood or plasma [3-5].
● The Influence of ADC Payloads Exposure on Antibody Concentration
Antibody drug conjugates (ADCs) are synthesized by conjugating a cytotoxic drug or “payload” to a monoclonal antibody, which may affect the distribution, metabolism, and clearance rate of antibodies in the body. In turns, it affects the effectiveness and safety of the drug. Generally, the clearance rate of antibodies increases upon binding to small molecule toxins, and the higher the DAR (Drug Antibody Ratio) value in ADC drugs, the faster the rate of ADC drug clearance. It suggests that drug coupling accelerates the process of antibody clearance, and a high ratio of drug binding may further accelerate this process, which is one of the key features of ADCs in tumor therapy [3-5].
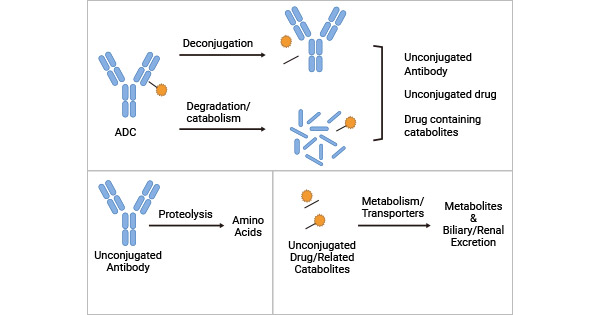
● The theoretical ADCs elimination pathways:
1. ADCs deconjugation or degradation/catabolism: ADCs may be degraded by enzymes in the body through metabolic pathways, resulting in ADCs elimination.
2. ADC deconjugation leads unconjugated antibody and unconjugated drug: the ADC payloads (drugs) separates from the antibody and loses its ability to bind to the antibody, i.e., the DAR becomes 0.
Figure 2. the theoretical ADC elimination pathways [5]
● The Evaluation of the Rate of Drug Loss from ADCs
The drug loss rate or drug-elimination rate is how fast the active ingredient (payload) is permanently removed from the body through various elimination pathways within an Antibody-Drug Conjugate (ADC). In ADCs, the drug’s active ingredient typically reaches its target by forming a covalent bond with the antibody. However, this process can result in drug loss, potentially compromising the drug’s targeting precision and efficacy. Moreover, excessive drug loss might lead to adverse reactions. Consequently, assessing the in vivo drug loss rate of ADCs plays a crucial role in the development and evaluation of safety and efficacy during ADC preclinical stages [6-8].
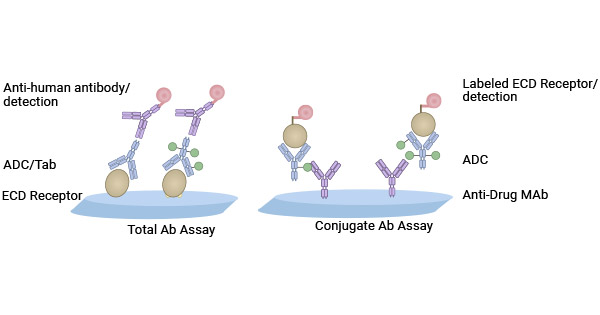
In Antibody-Drug Conjugates (ADCs), the significant differences of conjugated antibodies PK versus total antibody PK indicates a high variability in the rate of loss or degradation of the drug in ADC in vivo. Variations in antibody concentration changes versus ADC drug clearance indirectly suggests the stability of the drug in the bloodstream, because it affects the duration and effectiveness of the drug in the body [6-8].
In Antibody-Drug Conjugates (ADCs), the clearance rate of the drug tends to be faster than the total antibody concentration. The two processes—the clearance of drug and ADC itself together—reduce the drug concentration within the ADC. However, the total antibody concentration is influenced only by ADC clearance and unconjugated antibodies. If total antibodies disappear rapidly but ADC drug clearance is slower, it suggests a delayed drug release. This delay could result from a drug with a high protein-binding affinity or an impaired drug release mechanism [6-8].
Figure 3. ELISA Assay for Total Ab and Conjugate Ab [7]
A Highly Potent Tool for ADCs Pharmacokinetic (PK) Analysis
To fully support researchers and pharmaceutical companies in the pharmacokinetic analysis of DXD-ADC, CUSABIO has launched the PK research tool——DXD Monoclonal Antibody, with high specificity, stability and sensitivity, is capable of effectively determining the stability and release efficiency of DXD.
DXD Monoclonal Antibody can be used for plasma/serum kinetic analysis, drug binding and affinity determination, DAR value analysis and therapeutic evaluation of ADC drugs. This will accelerate the DXD-ADC drug development process and provide a basis for preclinical application and dosage optimization by evaluating the pharmacokinetic (PK) analysis of the new generation of ADCs!
● DXD Monoclonal Antibody Code: CSB-MA996977I2m
DXD antibody on SDS-PAGE under reducing (R) condition
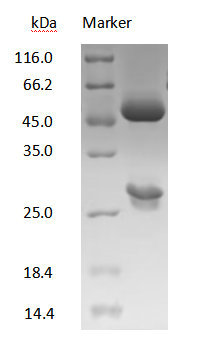
The purity of the protein is greater than 90%.
The Binding Activity of ADC-DXD(1) with Anti-DXD antibody
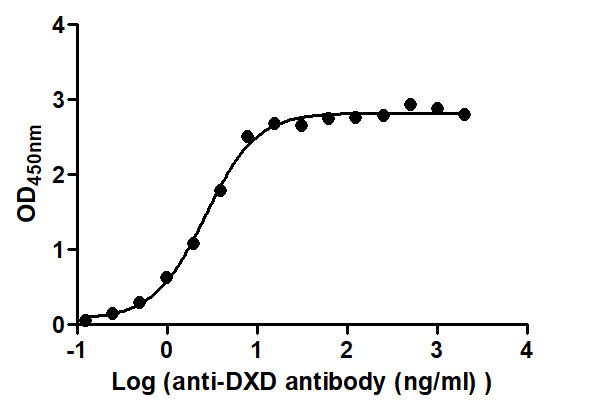
Activity: Measured by its binding ability in a functional ELISA. Immobilized ADC-DXD(1) at 2 μg/mL can bind Anti-DXD antibody, the EC50 is 2.298 to 3.054 ng/mL.
The Binding Activity of ADC-DXD(1) with Anti-DXD antibody
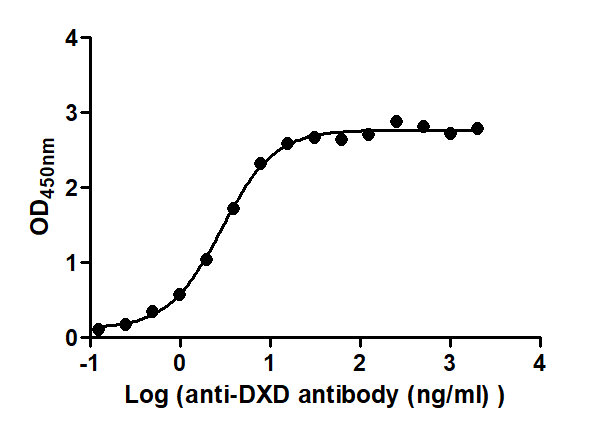
Activity: Measured by its binding ability in a functional ELISA. Immobilized ADC-DXD(2) at 2 μg/mL can bind Anti-DXD antibody, the EC50 is 2.566 to 3.233 ng/mL.
The Binding Activity of T-DXd(DS-8201) with Anti-DXD antibody
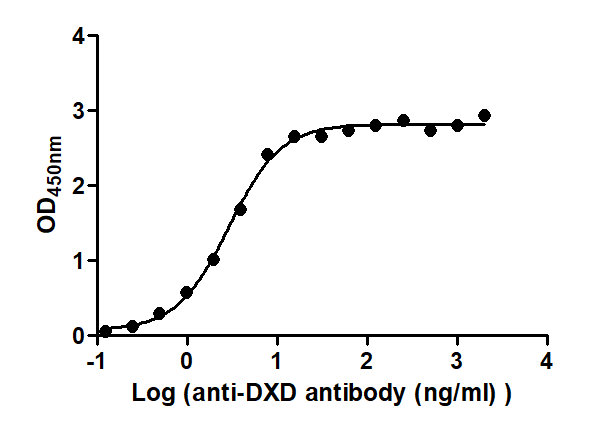
Activity: Measured by its binding ability in a functional ELISA. Immobilized T-DXd(DS-8201) at 2 μg/mL can bind Anti-DXD antibody, the EC50 is 2.529 to 3.340 ng/mL.
● DXD Monoclonal Antibody Code: CSB-MA996977I1m
DXD antibody on SDS-PAGE under reducing (R) condition
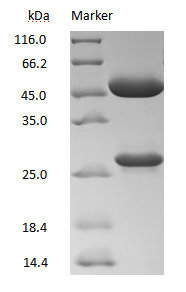
The purity of the protein is greater than 90%
The Binding Activity of ADC-DXD(1) with Anti-DXD antibody
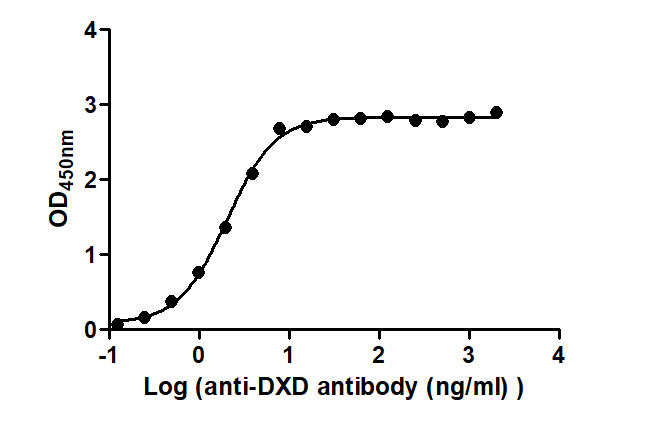
Activity: Measured by its binding ability in a functional ELISA. Immobilized ADC-DXD(1) at 2 μg/mL can bind Anti-DXD antibody, the EC50 is 1.840 to 2.253 ng/mL.
The Binding Activity of ADC-DXD(2) with Anti-DXD antibody
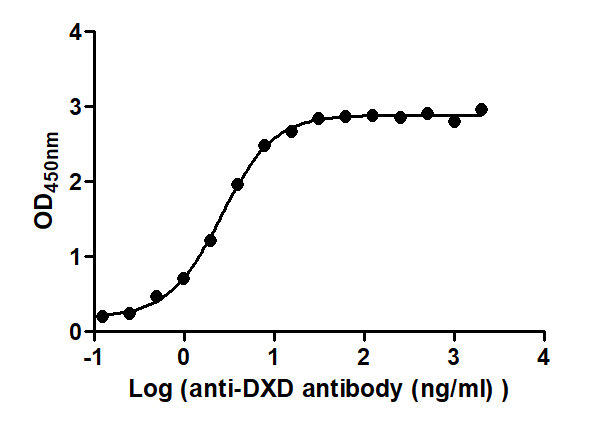
Activity: Measured by its binding ability in a functional ELISA. Immobilized ADC-DXD(2) at 2 μg/mL can bind Anti-DXD antibody, the EC50 is 2.365 to 2.835 ng/mL.
The Binding Activity of T-DXd(DS-8201) with Anti-DXD antibody
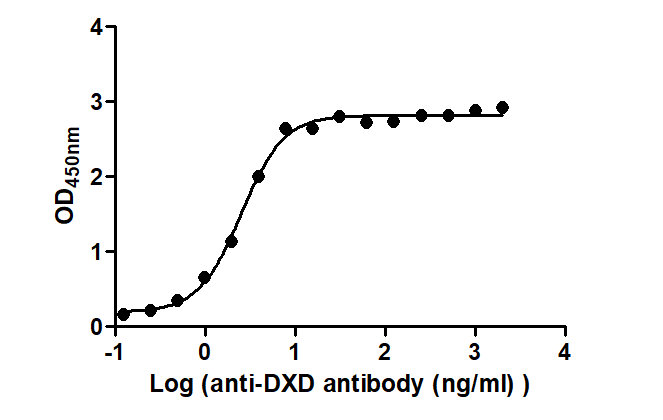
Activity: Measured by its binding ability in a functional ELISA. Immobilized T-DXd(DS-8201) at 2 μg/mL can bind Anti-DXD antibody, the EC50 is 2.225 to 2.851 ng/mL.
References
[1] https://xueqiu.com/8965749698/192610616
[2] Mahmood, Iftekhar. "Clinical pharmacology of antibody-drug conjugates." Antibodies 10.2 (2021): 20.
[3] Hedrich, William D., et al. "Antibody–drug conjugates: pharmacokinetic/Pharmacodynamic modeling, preclinical characterization, clinical studies, and lessons learned." Clinical pharmacokinetics 57 (2018): 687-703.
[4] Zhang, Donglu, et al. "Exposure-efficacy analysis of antibody-drug conjugates delivering an excessive level of payload to tissues." Drug Metabolism and Disposition 47.10 (2019): 1146-1155.
[5] Kamath, Amrita V., and Suhasini Iyer. "Preclinical pharmacokinetic considerations for the development of antibody drug conjugates." Pharmaceutical research 32 (2015): 3470-3479.
[6] Li, Hu, and Hongfeng Li. "A narrative review of the current landscape and future perspectives of HER2-targeting antibody drug conjugates for advanced breast cancer." Translational Breast Cancer Research 2 (2021).
[7] Lin, Kedan, Jay Tibbitts, and Ben-Quan Shen. "Pharmacokinetics and ADME characterizations of antibody–drug conjugates." Antibody-Drug Conjugates (2013): 117-131.
[8] Saad O M, Shen B Q, Xu K, et al. Bioanalytical approaches for characterizing catabolism of antibody-drug conjugates[J]. Bioanalysis, 2015, 7(13): 1583-1604.
CUSABIO team. Anti-Payload Antibody DXD: A Highly Potent Tool for ADCs Pharmacokinetic (PK) Analysis!. https://www.cusabio.com/c-21151.html












Comments
Leave a Comment