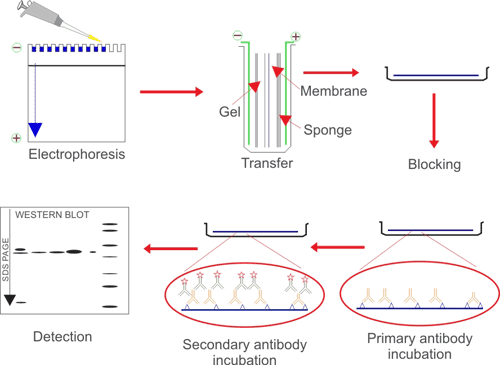
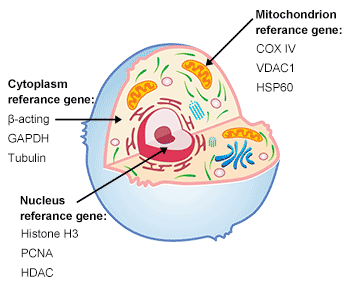
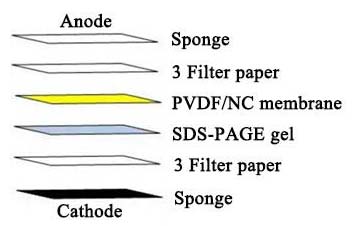
1. Protein Sample Preparation
1.2 Protein Sample Preparation
Generally, complex protein components are extracted from animal or plant tissues or cells, and the following principles should be observed during the extraction process:
a. Decide the appropriate extraction method based on the characters of individual protein.
b. Use the appropriate method to maximize the extraction of target protein.
c. Perform under low temperature and add protease inhibitors to prevent protein degradation.
d. Choose the appropriate protein lysate to maintain protein solubility.
Store Protein samples at -80℃, avoid repeated freezing and thawing, detect as soon as possible.
Preparation of lysate from cell culture
a. After the cell confluence reaches 80%, place the cell culture dish on ice and wash the cells with ice-cold PBS for 3 times.
b. Prepare lysates which containing protease inhibitors. The commonly used protease inhibitors are shown in the table below (Table 2). Appropriate protease inhibitors should be selected according to the experimental requirements. The most commonly used protease inhibitor is PMSF (working concentration is 1 mM), which is highly toxic, so it should be self-protected when used. Its half-life in water is extremely short, so it should be added before use.
c. Add 1 mL of protein lysate containing protease inhibitor to a 10 cm culture dish, shake gently, and lyse on ice for 15-30 min.
d. Scrape adherent cells off the dish using a cold plastic cell scraper, then gently transfer the cell suspension into a 1.5 mL EP tube, then place the tube on ice. Bubbles should be avoided at this time.
Notes:
The collected cells can also be fully lysed by sonication. Place the ultrasound probe in the middle of the sample lysate, but do not touch the tube wall or tube bottom for ultrasound. The sonicator we used is Scientz JY92-IIN, with 10% power (650 W), over 2 sec, stop for 3 sec. Basically, the intracellular suspension of 1mL should be sonicated for 10-25 cycles.
e. Centrifuge at 12000 rpm for 10-15 min at 4℃.
f. Gently aspirate the supernatant to another fresh tube and place on ice for later use. Be careful not to absorb impurities such as lipids floating in the upper layer.
g. After protein quantification, add appropriate amount of 6 × sample loading buffer, and boil at 95℃ for 5 min, then centrifuge at 12000 rpm for 30 sec, lastly, store at -20℃.
Sample preparation notes:
All steps must be operated at low temperature! Low temperature! Low temperature!
a. For the cells grown in suspension, collect by centrifugation at 2500 rpm for 3 min, followed by cell washing and lysis procedures.
b. For drug-treated cells, especially samples from apoptosis related studies, media supernatants should also be collected.
c. It is not recommended to use protease to digest and collect cells. Because it may introduce protein impurities or cause damage to some certain proteins, especially the membrane surface proteins, to interfere the experimental results.
d. A viscous transparent gel may appear in the lysate. The transparent gel is a genomic DNA component. Take the supernatant for experiments. However, when the target protein is tightly bound to the genome, the gel needs to be ultrasonically disrupted or syringe-sucked, then take supernatant for subsequent experiments to avoid protein loss.
e. PMSF is unstable in aqueous solution, usually it degrades by half in 30 min. The rate of loss of activity increases with the increase of pH value, and the deactivation rate at 25℃ is higher than 4℃. When the sample is processed for more than 1 h, it needs to be added once more.
f. Pay attention to the influence of cell state and the number of cell passages. Heterogeneity exists in cancer cells of different algebras, so the cell morphology, migration and invasion ability may change, thereby make some gene expression change as well.
On one hand, due to the certain heterogeneity of the cells themselves, after a period of cultivation, the overall characteristics of the cells are gradually changed in a way of survival of the fittest.
On the other hand, in the process of cell culture, due to changes in culture conditions or the presence of external stimuli, such as replacement of culture reagents, digestion and passage, cell contamination, and some chemical and physical stimuli, the expression of related genes in cells may be affected. Ultimately affect the experimental results.
When using tumor cells for experiments, it should be preserved first, and try to use relevant cells in the same algebra to carry out relevant experimental research to avoid the occurrence of cell heterogeneity due to excessive passage times, and ultimately lead to inconsistencies in experimental results.
Preparation of lysate from tissues
a. Collect fresh samples and wash with saline or PBS, then cut into appropriate sizes. You can use a 1-2 mL homogenizer for tissue homogenate on ice, or add liquid nitrogen for grinding. It is recommended to use liquid nitrogen grinding, for the tissue block is not easily damaged and there is frictional heat generation during the homogenization process.
b. Prepare the lysate containing protease inhibitor.
c. Add appropriate amount of lysate containing protease inhibitor (50 mg/500 μL) into the grinded tissue sample, and place the tube on ice for 15-30 min for lyse, meanwhile, intermittently mix to fully lyse.
Notes:
To ensure adequate lysis of tissue cells, sonication is recommended. Adjust the ultrasound system to the appropriate frequency and power (the ultrasonic power should not be too large, and set the ultrasonic intermittent to prevent the ultrasonic probe from overheating). Place the ultrasonic probe in the middle of the sample lysate, but do not touch the tube wall or the bottom of the tube, for ice bath ultrasound.
d. Centrifuge at 12000 rpm for 10-15 min at 4℃.
e. Remove the EP tube gently, and absorb the supernatant into a fresh tube. Be careful not to absorb impurities such as lipids floating in the upper layer, then place on ice for later use.
f. Centrifuge at 12000 rpm for 10-15 min at 4℃.
Notes:
The tissue sample must be cleaned, so remove the blood vessels, and wash the blood away to avoid interference of IgG in the sample.

Fig. 2. The processes of lysate preparation
1.3 Selection of Protein Lysate
For most samples, RIPA lysis buffer can be used for rapid cell lysis.
Table 1. The components of RIPA lysis buffer
|
RIPA Lysis Buffer
|
|
Tris-HCl
|
50 mM
|
|
NaCl
|
150 mM
|
|
EDTA
|
1 mM
|
|
SDS (W/V)
|
0.1% (W/V)
|
|
Sodium deoxycholate
|
1% (W/V)
|
|
Triton X-100
|
1% (V/V)
|
|
Appropriate protease inhibitors can be added to the RIPA lysis buffer according to the purpose of experiment.
|
The main components of the protein lysate and their effects are as follows:
Buffer
A buffer with a certain pH range could provide a stable environment for proteins and increase protein solubility as well. The buffers of Tris-HCl or HEPES, pH 7.4 with similar physiological pH are commonly used. The buffer of Tris-HCl (pKa = 8.1) has a pH range of 7.0-9.2, which is sensitive to temperature. The pH value range of HEPES (pKa = 7.55) is 6.5-8.5.
Saltion
The appropriate salt ion concentration could maintain protein solubilization. The selection of 150 mM NaCl in an approximate physiological state will not affect the disruption of proteins and protein interactions.
Chelating agent
Chelate metal ions are used to prevent protein extracts from becoming too viscous, resulting in reduced solubility. In addition, chelating agents can also interact with certain enzymes to inhibit enzyme activity.
Reducing agent
The addition of a certain amount of reducing agent could protect the free sulfhydryl groups on the protein from oxidation, thereby avoiding protein aggregation or denaturation. Theβ-mercaptoethanol and dithiothreitol (DTT) are commonly used as reducing agents, the latter one is more powerful than the former one. Usually, theΒ-mercaptoethanol is volatile and will be oxidized in a short time after being added to the buffer, which could affect the activity of the protein, and its working concentration is 5-20 mM/L. The DTT has a stronger reducing ability, which can form a stable intramolecular disulfide bond after oxidation without any affect to the protein sulfhydryl group. Its working concentration is 0.5-1 mM/L. Basically, DTT is recommended for long-term storage, but the DTT solution is not stable and needs to be used right after it was ready.
Detergent
The detergent is a kind of surfactant, and the hydrophobic segment of the surfactant molecule is inserted into the phospholipid bilayer of the membrane, thereby changing its permeability and ultimately destroying the membrane structure. Therefore, the strength of the surfactant directly determines the ability of lysating cells. The surfactants used in the lysate can be mainly divided into two major kinds: anionic surfactants and nonionic surfactants. Commonly used surfactants are as follows:
-
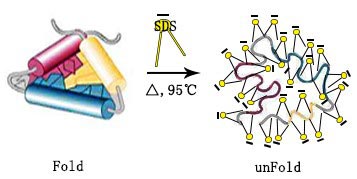
SDS: The anionic surfactant, has a strong destructive power, which can basically dissolve all proteins and destroy their natural conformational structure. SDS binds with protein in a ratio of 1.4:1, which can effectively cover the charge of the protein itself. The critical micelle temperature of SDS is a little high, so precipitation could occur at low temperatures, and the precipitation will bemore obvios in the presence of potassium salts. In addition, the stronger the ionic strength of the solution, the lower the critical micelle concentration of the ionic detergent, making the protein more soluble.
-
NaDOC: A kind of ionic surfactant, which is weaker than SDS.
-
Triton X-100: A kind of non-ionic surfactant. It can destroy the interaction between protein and lipid, but it does not denature the protein, or break the connection between protein and protein neither. It can preserve the natural conformation of the protein. It has a lower critical micelle concentration and two-phase separation can be observed at 64℃.
-
NP-40: A kind of non-ionic surfactant, it has weak damage to the nuclear membrane, however, it has strong binding ability to proteins, and could ensure sufficient solubility and structural stability of the protein, so it is particularly suitable for dissolution of membrane proteins under non-deformation conditions.
-
Tween 20: A kind of mild non-ionic surfactant with poor ability for protein solubilization, which does not destroy protein structure and is not a common component of protein lysates.
The selection of detergent depends on the nature of the protein to be extracted and the purpose of the experiment. There are many factors should be considered when choosing the detergent, including fully lysating the cells and dissolving the protein, for the state of the extracted protein (denatured or retained in a natural state).
Protease inhibitors
A large amount of protease is released when cells and tissues are destroyed during protein extraction process. In order to inhibit protease activity, the samples must be kept at low temperature and an appropriate amount of protease inhibitor should be added to prevent degradation of the protein of interest.
Table 2. Commonly used protease inhibitors
|
Protease inhibitor
|
Function
|
Working concentration
|
Characters
|
|
PMSF
|
Serine proteases inhibitor
Cysteine proteases inhibitor
|
0.5-1 mM
|
PMSF has a short half-life in water and needs to be added shortly before use. Very toxic, should pay attention to self-protection during experimental operation
|
|
APMSF
|
Serine proteases inhibitor
|
0.4-4 mM
|
-
|
|
Pepstatin
|
Aspartyl proteases inhibitor
|
1 μM
|
Store at -4℃ for 1 week, -20℃ for up to 1 month; avoid repeated freeze/thaw cycle.
|
|
Leupeptin
|
Serine proteases inhibitor and Cysteine proteases inhibitor
|
10-100 μM
|
Store at -4℃ for 1 week, -20℃ for up to 1 month; avoid repeated freeze/thaw cycle.
|
|
Aprotinin
|
Serine proteases inhibitor
|
0.01-0.03 μM
|
Store at -4℃ for 1 week, -20℃ for up to 1 month; avoid repeated freeze/thaw cycle.
|
|
Na3VO4
|
Phosphatases inhibitor
|
1 mM
|
Need to be activated. Add acid to adjust the pH to 10 after dissolving, and heat to boil to colorless, cool at room temperature, then adjust the pH to 10 again. Repeat the steps until the solution remains colorless and the pH is stable at 10, aliquot and store at -20 ° C.
|
|
NaF
|
Phosphatases inhibitor
|
10-20 mM
|
-
|