In fact, choosing a right antibody could be difficult even for a researcher with abundant experience in doing various experiments. When you are working with a relatively uncommon model system or the protein studied has not been well characterized, there may be just a limited number of antibodies commercially available or even no one at all. When you are studying a well-characterized protein in a common model system, you may have another problem -- there are so many antibodies supplied by so many antibody manufactures and distributors. In this case, you have a lot of choices, but it's also not easy to make the correct decision.
The good news is that there are many methods to simplify the selection process. In this article, we'll give you some suggestions. This article mainly covers these topics:
1. The selection of specificity
The selection of specificity mainly needs considering four aspects: the specificity of proteins, the specificity of species, the specificity of experimental methods, and the specificity of the label.
1.1 The specificity of proteins
What exactly is protein specificity? Generally, protein specificity refers to the ability of a protein's binding site to bind specific ligands. An example of a protein-ligand pair is the antibody-antigen system, whose binding activity can be described as highly specific. After understanding what is protein specificity, it would be easier to understand antibody specificity. Each individual antibody protein is capable of binding specifically with one unique epitope on the antigen due to the unique antigen binding site at the tip of the variable region on the Y-shaped antibody protein. This specificity allows precise detection of a target antigen.
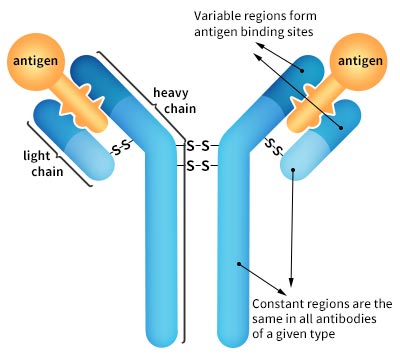
Pic. 1 Antibody Structure
When choosing an antibody according to the protein detected, you should know these details:
a. If the recombinant protein is not expressed in full length, it is necessary to confirm whether the antigen binding site falls within the sequence range of the recombinant protein.
b. For endogenous proteins, it's better to know their splicing and modification. For proteins with special phenotype, it's required to perform sequence alignment to check the species cross-reactivity by comparing immunogen sequence with the sequence of protein you're interested in.
c. The detection of phosphorylated proteins requires the identification of specific phosphorylation sites. Different phosphorylation sites mean that there may be different mechanisms which should not be generalized.
1.2 The specificity of species
Homologous proteins vary more or less from one species to another.
a. Most commercial antibodies are produced using recombinant protein or peptide derived from human protein sequence, so they could cross-react with other species based on sequence homology. You need to look at the species reactivity stated on the antibody data sheet.
b. For some rare species, it's difficult to find antibodies for them. In this case, you should check the sequence alignment to choose antibodies generated with homologous sequence, but generally the manufacturers will not accept complaints about the quality. It will be easier to choose the right antibody if you can obtain a free antibody sample from the supplier.
1.3 The specificity of the experimental method
There are a lot of experiments that involve the use of antibodies. In different methods, due to the differences in sample preparation and target protein content, there are different requirements on the epitope and antibody titer.
1.4 The specificity of the label
Common experiments such as WB and IHC generally do not require labeled primary antibody but use labeled secondary antibody. However, some experiments that are based on instrument analysis may use labeled primary antibody, such as FC. In this case, it's required to understand the spectrum of fluorescence that can be detected by the instrument used, and select the appropriate label according to different parameter requirements. In immunofluorescence double labeling experiments, different fluorescent labels should be selected and matched.
2. The selection of antibody depends on the experiment/application
2.1 How to choose an antibody for WB?
Many people suggest that you should use monoclonal antibodies as the primary antibody in WB. Currently, the polyclonal antibody production technique is as mature as the monoclonal antibody production technique. So, many polyclonal antibodies also perform well in WB. In addition to primary antibodies and secondary antibodies, WB also involves the use of a loading control antibody, which helps confirm that protein loading is the same across the gel. Loading controls are usually proteins that are expressed at high levels in the cell type you studied. Last but not least, no matter what experiment you are doing, it's recommended to refer to the relative literature to choose the right antibody.
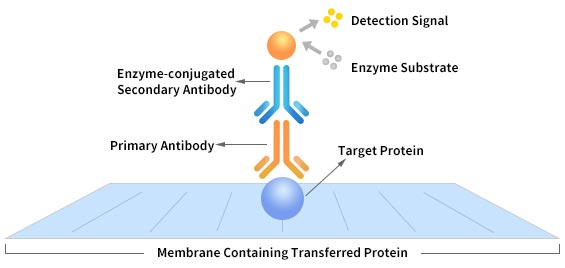
Pic. 2 Western Blot
2.2 How to choose an antibody for IP/ChIP?
In IP, antibodies bind to the natural form of the target protein. So, the best antibodies for IP are those produced by using purified natural proteins or by using purified recombinant proteins. It's better not to use antibodies produced by using synthetic peptides for the reason that the regions recognized by this type of antibodies may be deeply hidden in the heart of the target protein.
There is not much difference between ChIP and IP. The only problem is that the experiment may fail if the region of the target protein that is recognized by antibodies is the same as the region of the target protein that binds to DNA.
2.3 How to choose an antibody for IF/IHC?
In IF/IHC, there is an important step called fixation that is to retain cellular and subcellular structure. Fixation can denature and immobilize proteins, meaning that these proteins become different from their natural forms but are also unlike linear structures seen in WB. Thus, for IF/IHC, the antibodies of choice are those produced by using purified recombinant proteins or by using synthetic peptides (peptides on the surface of protein).
2.4 How to choose an antibody for FC?
FC can be classified into two types: live-cell FC and 'fixed-cell' FC. For live-cell FC, you'd better choose antibodies produced by using natural proteins or recombinant proteins; for 'fixed-cell' FC, the antibodies used are the same as those used in IF/IHC. In FC, there are direct labeling and indirect labeling. Indirect labeling is less accurate than direct labeling. So, when doing FC, you'd better choose fluorescently-labeled antibody; if there are no labeled antibody against the protein of interest, you can then choose indirect labeling, in which secondary antibody labeled with a fluorophore is needed.
3. The selection of primary antibody and secondary antibody
3.1 How to choose primary antibody?
a. Confirm the name of the antibody. Full name, abbreviation, alternative names, isotype, and other details should be taken into consideration.
b. Confirm the type of the experiment/application. ELISA, WB, IHC, ICC, FACS, or others? Generally, the antibody data sheet will list the applications that the antibody has been validated in. If the antibody data sheet does not mention a specific application, it does not automatically mean that the antibody is not appropriate for the application, but it only means that the antibody hasn't been validated in the application. But generally, you'd better choose the right antibody according to validation information listed in the product data sheet.
c. Confirm the species of the sample. Human, mouse, rat, sheep, fish, or goat? Generally, the antibody data sheet includes the species reactivity information. With the information, you can tell whether the antibody would work for your experiment.
d. Structural properties of the target protein. Understanding the structural properties of target protein will help you choose the right antibody. This structural information includes structural domains of the target protein, whether proteins will be denatured or not during sample preparation, changes in the conformation of proteins, and other factors that may affect immune affinity reactions.
e. The selection of monoclonal antibody and polyclonal antibody. The market is dominated by polyclonal antibodies. Compared with polyclonal antibodies, monoclonal antibodies have higher specificity but lower affinity, leading to relatively lower antigen detection sensitivity. Polyclonal antibodies have lower specificity but higher affinity, so their sensitivity is higher. The use of polyclonal antibodies can increase the risk of non-specific staining, which can be avoided by blocking or other steps.
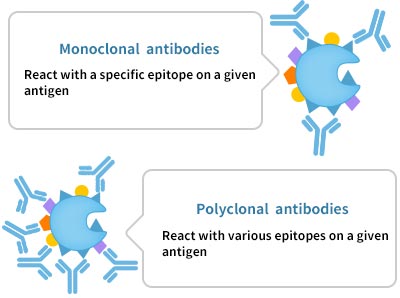
Pic. 3 Monoclonal Antibody VS. Polyclonal Antibody
3.2 How to choose secondary antibody?
a. Species or source. Choose a secondary antibody basing on the species of the primary antibody. For instance, if the primary antibody was raised in mouse, the secondary antibody should react to mouse antibodies.
b. The selection of the label. There are different labels such as HRP, Biotin, and fluorescein. Generally, SP three-step method uses the Biotin-labeled secondary antibody, to react with SP, while fluorescein staining uses secondary antibody labeled with different fluoresceins such as rhodamine, FITC, Cy3, etc.
4. FAQs
When you buy an antibody, the antibody supplier/manufacturer typically provides additional technical support, which is very important. In the past, some customers who bought antibodies from our company Cusabio sought technical support. Here are some of the most frequently asked questions.
4.1 Are antibodies for FC the same with antibodies for IHC?
They're not necessarily the same antibodies. For FC, there are direct and indirect labeling. For direct labeling, the antibody is usually FITC, TRITC, or PE-labeled. If you happen to do IHC and want to use the fluorescein-labeled antibody for direct labeling, then you can use the same antibody. If you want to use other labels in IHC, or indirect labeling, it may have trouble because fluorescein labels may affect the binding of the secondary antibody to the primary antibody.
4.2 Why does my antibody perform very well in WB but fail to work in other experiments such as IF and IHC?
First of all, congratulations that you have a good WB antibody. After all, it's not easy to get good WB antibodies. Secondly, your antibody may be produced by using synthetic peptides. As mentioned above, antigenic peptides recognized by your antibody may happen to be hidden in the heart of the target protein so that your antibody can only recognize the linear protein in WB.
4.3 How to choose antibodies for IHC?
First, it's needed to decide whether to choose a monoclonal or polyclonal antibody. The differences between monoclonal antibodies and polyclonal antibodies have been summarized above. If your experiment requires high specificity, monoclonal antibodies can be a better choice. If the positive signal is weak, try polyclonal antibodies. Polyclonal antibodies are often raised in rabbits, while monoclonal antibodies are often raised in mice. Of course, there are antibodies raised in other animals.
Second, it's important to understand which species the primary antibody can react with. This information is often stated in the antibody data sheet.
Third, you should pay attention to the source of the primary antibody. For example, when you perform IHC to detect p53 in human cancer tissue and use anti-p53 antibody raised in the mouse as the primary antibody, then you must choose an anti-mouse secondary antibody such as goat anti-mouse antibodies and rabbit anti-mouse antibodies. In a word, the primary antibody and the second antibody should match.
Finally, the antibody data sheet generally includes information about applications that the antibody can apply to. If the data sheet points out that the antibody cannot be used in IHC, don't choose it. Even if the data sheet highlights that the antibody can be used in IHC, there are cases that the antibody does not work well in IHC. Validating the antibody yourself can help you get a successful experiment result.
4.4 Are WB secondary antibodies and IF secondary antibodies the same?
Apparently, not! IF antibodies are used for the immunofluorescent test and are labeled with FITC, PE, or other labels that are activated by ultraviolet light, and the signal is detected by fluorescence microscopy. WB antibodies are generally labeled with HRP, alkaline phosphatase or other molecules that can react with chromogenic substrates (such as OPD\TMB), and the result can be observed directly by naked eye or detected by instruments for chemiluminescence.
4.5 Can ChIP antibodies be used in WB?
Not necessarily. Generally speaking, ChIP antibodies have higher requirements on purity, specificity, and other parameters. So, ChIP antibodies usually can be used in WB. However, ChIP antibodies recognize conformational epitopes, but not linear epitopes. This type of ChIP antibodies cannot be used in WB.
4.6 What if the antibody data sheet does not mention a specific application?
Generally, antibodies will be validated in WB, IP, IF, and IHC. So, you'd better not use the antibody to do an experiment that is not mentioned in the antibody data sheet.
4.7 Can ELISA antibodies be used for WB?
For WB, the dilution factor of antibodies is generally 1:1000; for IF/IHC, 1:100; for ELISA, 1:10000. If an antibody is used for ELISA, it generally means that the antibody titer is not high. This does not mean that all antibodies used in ELISA cannot be used in WB. If the antibody data sheet says that the dilution factor for ELISA is 1:1000, then you can try 1:100 to do WB.







Comments
Leave a Comment