The difference between primary antibody and secondary antibody
What is the difference between a primary antibody and a secondary antibody? This is a frequently asked question by green hands in the laboratory. Here we will answer this question from five angles: binding capability, use & label, source, clonality, and pre-adsorption.
1. Binding Capability
Antibodies (Ab) are extensively used for research. Depending on their binding capability, antibodies can be classified into two types: primary antibodies and secondary antibodies. Specifically, primary antibodies are antibodies that bind to the protein or antigen (Ag) or any substance you study, whereas secondary antibodies are those binding to primary antibodies. Look at the Fig. 1 below:
Fig. 1 Primary Ab VS. Secondary Ab
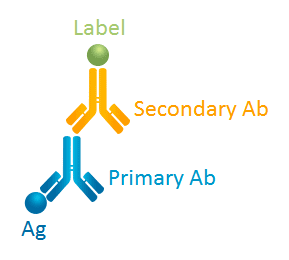
If you have read our previous article What is an antibody? you will acquire a good knowledge of antibody structure, and then you will find it easier to understand that the primary antibody's Fab domain binds to an antigen and exposes its Fc domain to the secondary antibody, and thus the secondary antibody's Fab domain binds to the primary antibody's Fc domain.
Since the Fc domain is the same in all antibody molecules of the same class in a given species, one type of secondary antibody is capable of binding to many types of primary antibodies. For example, when antibodies of the IgG class raised in mice are used as a primary antibody, an antibody that targets mouse IgG Fc domain and is not raised in mice can be used as a secondary antibody.
2. Use & Label
In immunoassays, a primary antibody is always needed to bind to the target antigen, but a secondary antibody is not necessarily used. Let's take direct ELISA and indirect ELISA as an example. Direct ELISA involves direct labeling, meaning that the primary antibody is directly labeled with an enzyme for enzymatic reaction and signal detection, and there is no need for a secondary antibody. Indirect ELISA does not label the primary antibody directly, but instead, it labels the secondary antibody with an enzyme, as shown in Fig. 2 below.
Fig. 2 Direct ELISA VS. Indirect ELISA
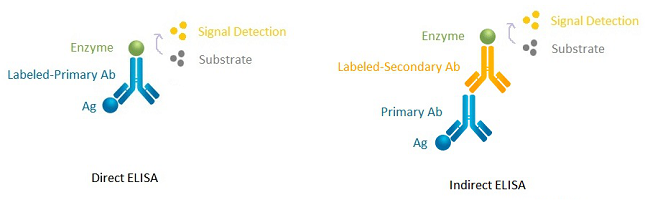
Whether to label the primary antibody (direct labeling) or the secondary antibody (indirect labeling) depends on your experiment. Either direct labeling or indirect labeling has its advantages and disadvantages. Direct labeling is generally associated with a simple protocol that saves time and reagents, has no risk of cross-reactivity from secondary antibody, but lacks signal amplification and flexibility and may result in high background. Indirect labeling involves signal amplification, is much flexible as the same secondary antibody can react with multiple primary antibodies, but has a relatively complex protocol and cross-reactivity risk.
3. Source
The source of antibodies is an important aspect that needs to consider. The host species of the primary antibody should be different from the species of your sample, to avoid cross-reactivity from the secondary antibody with sample immunoglobulins. In addition, the host species of the secondary antibody should be different from the host species of the primary antibody, since the secondary antibody should be directed against the species of the primary antibody.
For example, if you study a mouse sample, you will need a primary antibody that is generated in animals other than mouse, such as those generated in rabbit. Then, if you use a rabbit antibody directed against mouse as a primary antibody, you will need a secondary antibody that is generated in animals other than rabbit, such as those generated in goat.
To enable detection of target antigen, the secondary antibody should not only have specificity for the species of the primary antibody, but also have specificity for the isotype of the primary antibody. For instance, if the primary antibody is an IgG1 antibody raised in rabbit, you will need a secondary antibody that can react with rabbit IgG1 antibody and is not generated in rabbit.
4. Clonality
The primary antibody can be either a monoclonal antibody (mAb) or polyclonal antibody (pAb).
Let's first compare monoclonal antibodies and polyclonal antibodies. Monoclonal antibodies recognize only one epitope on an antigen, while polyclonal antibodies recognize multiple epitopes on an antigen. Due to this, monoclonal antibodies and polyclonal antibodies have different properties and uses. Monoclonal antibodies have high specificity and high reproducibility, causing less background from staining as compared with polyclonal antibodies. However, due to their high specificity, monoclonal antibodies may fail to work when the target epitope is changed by chemical treatment of the antigen. By contrast, polyclonal antibodies have high affinity, are tolerant of minor changes of the antigen, and allows more robust detection. But polyclonal antibodies have higher batch-to-batch variability, and are more likely to create high background from staining.
If your experiment requires high specificity, using monoclonal antibodies as primary antibodies can be a good choice. If the positive signal is weak, try polyclonal antibodies. Since polyclonal antibodies recognize multiple epitopes, they are more likely to give better results in immunoprecipitation (IP) and chromatin immunoprecipitation (ChIP). But if you want to probe specific domains of antigen, polyclonal antibodies are not useful, and monoclonal antibodies as primary antibodies could be better.
The secondary antibody can be either a monoclonal antibody or a polyclonal antibody that binds to the primary antibody or its fragments. There are different forms of secondary antibodies. First, a secondary antibody can be directed against the whole molecule of the primary antibody. For instance, a primary antibody is generally IgG isotype. Then, you will need an anti-IgG H&L (Heavy & Light chains) antibody as a secondary antibody, which reacts with both the heavy and light chains of the primary IgG antibody. In addition, this anti-IgG H&L antibody also reacts with other immunoglobulin classes such as IgM and IgA of the species of the primary antibody, because all immunoglobulins in a given species have the same light chains. Second, a secondary antibody can be specific for the Fab region of the primary antibody. Likewise, this form of secondary antibody also reacts with both heavy and light chains, and therefore reacts with other immunoglobulin classes with the same light chains. Third, a secondary antibody can be specific for the Fc region of the primary antibody, meaning that it reacts only with the heavy chain. So this secondary antibody only reacts with one immunoglobulin class, such as IgG or IgM. Finally, a secondary antibody can be specific for the light chain of the primary antibody, so it will react with all immunoglobulin classes with the same light chain.
5. Pre-adsorption
Secondary antibodies sometimes need an extra purification process, known as pre-adsorption, to increase specificity and minimize non-specific. During pre-adsorption, the solution containing secondary antibodies is passed through a column matrix containing immobilized serum proteins or immunoglobulins from potentially cross-reactive species (such as the species of the sample). Through this process, non-specific antibodies in the solution will be removed, while highly specific secondary antibodies are left. For example, if you are working with mouse tissues, choose a secondary antibody that has been adsorbed with mouse serum or mouse IgG.
Pre-adsorbed secondary antibodies produce less non-specific binding and therefore are less likely to create non-specific background. When you determine protein expression in multiple labeling experiments or when you study immunoglobulin-rich tissues or cells, you are recommended to use a pre-adsorbed secondary antibody.
In summary, there are several differences between primary antibodies and secondary antibodies. Firstly, primary antibodies and secondary antibodies have different binding capacity. Primary antibodies bind to the antigen detected, whereas secondary antibodies bind to primary antibodies, usually their Fc domain. Secondly, primary antibodies are always needed in immunoassays, whereas secondary antibodies are not necessarily needed, which depends on experimental method (direct or indirect labeling). Thirdly, the species primary antibodies are generated in is not the same species as the sample, and the species secondary antibodies are generated in is different from the species source of primary antibodies. Fourthly, both primary antibodies and secondary antibodies can be either monoclonals or polyclonals, but there are different things to consider in selection of their clonality. Finally, secondary antibodies sometimes need to be pre-adsorbed, while there is generally not such a need for primary antibodies.
Now, you may get a better understanding of the difference between primary antibodies and secondary antibodies and will find it easier to select the right antibodies for your experiments. Choosing the right antibody is never an easy task. If you've chosen an antibody that is unsuitable for your experiment, you may waste money, time, and precious samples. To simplify the selection of antibody, you can continue to read another article on our site: How to Choose an Antibody for Scientific Research?
Cite this article
CUSABIO team. The difference between primary antibody and secondary antibody. https://www.cusabio.com/c-15039.html






Comments
Leave a Comment