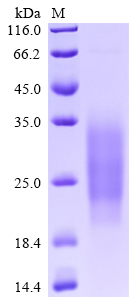
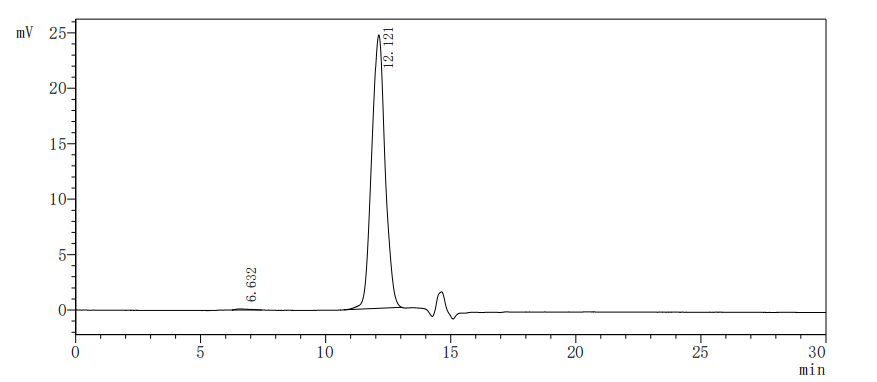
The recombinant human SIGIRR protein is synthesized through the expression of the target gene encoding the 1-118aa of the human SIGIRR in mammalian cells. The target gene is co-expressed with the C-terminal 6xHis-tag gene via the plasmid. The resulting SIGIRR protein is subjected to affinity chromatography purification, getting a purity exceeding 95% as determined by SDS-PAGE.
The human SIGIRR (TIR8) is a member of the TLR superfamily and regulates immune responses. Structurally, SIGIRR is characterized by a single immunoglobulin (Ig) extracellular domain, a transmembrane domain, and an intracellular Toll/IL-1 receptor (TIR) domain, which is essential for its function as a negative regulator of TLR and interleukin-1 receptor (IL-1R) signaling pathways that modulate inflammatory responses [1][2][3].
SIGIRR is primarily expressed in epithelial tissues, including the kidney, colon, and gastrointestinal tract, where it serves to maintain homeostasis and prevent excessive inflammation [3][4][5]. Its expression is particularly significant in intestinal epithelial cells (IECs), where it regulates the innate immune response by inhibiting the activation of pro-inflammatory signaling pathways such as those mediated by MyD88-dependent signaling [6][7]. This regulatory function is critical in preventing tissue damage and maintaining the balance of immune responses during infections and inflammatory conditions [8][9].
Research has demonstrated that SIGIRR negatively regulates IL-1 signaling by sequestering key signaling molecules such as IRAK and TNF receptor-associated factor (TRAF), from the receptor complex [10][11][12]. This action effectively dampens the recruitment of these components to the receptor, thereby attenuating downstream signaling cascades that lead to inflammation [12][13]. Furthermore, SIGIRR is involved in various pathological conditions, including bacterial infections and Kawasaki disease, where it regulates endothelial apoptosis and inflammation [14][15].
References:
[1] D. Li, X. Zhang, & B. Chen. Sigirr participates in negative regulation of lps response and tolerance in human bladder epithelial cells, BMC Immunology, vol. 16, no. 1, 2015. https://doi.org/10.1186/s12865-015-0137-5
[2] F. Tian, J. Lei, Y. Ni, D. Zhong, N. Xie, J. Ma, et al. Regulation of cd18 stability by sigirr‐modulated ubiquitination: new insights into the relationship between innate immune response and acute lung injury, Febs Journal, vol. 290, no. 10, p. 2721-2743, 2022. https://doi.org/10.1111/febs.16708
[3] F. Tian, Q. Lu, J. Lei, Y. Ni, N. Xie, D. Zhong, et al. Negative effects of sigirr on traf6 ubiquitination in acute lung injury in vitro, Journal of Immunology Research, vol. 2020, p. 1-9, 2020. https://doi.org/10.1155/2020/5097920
[4] M. Watson, D. Costello, D. Carney, K. McQuillan, & M. Lynch. Sigirr modulates the inflammatory response in the brain, Brain Behavior and Immunity, vol. 24, no. 6, p. 985-995, 2010. https://doi.org/10.1016/j.bbi.2010.04.002
[5] X. Chen, Y. Zhao, X. Wu, & G. Qian. Enhanced expression of single immunoglobulin il-1 receptor-related molecule ameliorates lps-induced acute lung injury in mice, Shock, vol. 35, no. 2, p. 198-204, 2011. https://doi.org/10.1097/shk.0b013e3181f226f3
[6] H. Sham, E. Yu, M. Gulen, G. Bhinder, M. Ståhl, J. Chan, et al. Sigirr, a negative regulator of tlr/il-1r signalling promotes microbiota dependent resistance to colonization by enteric bacterial pathogens, Plos Pathogens, vol. 9, no. 8, p. e1003539, 2013. https://doi.org/10.1371/journal.ppat.1003539
[7] J. Allaire, A. Poon, S. Crowley, X. Han, N. Moore, M. Stahl, et al. Interleukin-37 regulates innate immune signaling in human and mouse colonic organoids,, 2020. https://doi.org/10.21203/rs.3.rs-126899/v1
[8] R. Gopal, D. Birdsell, & F. Monroy. Regulation of toll‐like receptors in intestinal epithelial cells by stress and toxoplasma gondii infection, Parasite Immunology, vol. 30, no. 11-12, p. 563-576, 2008. https://doi.org/10.1111/j.1365-3024.2008.01055.x
[9] F. Riva, E. Bonavita, E. Barbati, M. Muzio, A. Mantovani, & C. Garlanda. Tir8/sigirr is an interleukin-1 receptor/toll like receptor family member with regulatory functions in inflammation and immunity, Frontiers in Immunology, vol. 3, 2012. https://doi.org/10.3389/fimmu.2012.00322
[10] M. Gulen, Z. Kang, K. Bulek, Y. Wan, T. Kim, Y. Chen, et al. The receptor sigirr suppresses th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mtor kinase activation, Immunity, vol. 32, no. 1, p. 54-66, 2010. https://doi.org/10.1016/j.immuni.2009.12.003
[11] T. Bauman, A. Becka, P. Sehgal, W. Huang, & W. Ricke. Sigirr/tir8, an important regulator of tlr4 and il-1r–mediated nf-κb activation, predicts biochemical recurrence after prostatectomy in low-grade prostate carcinomas, Human Pathology, vol. 46, no. 11, p. 1744-1751, 2015. https://doi.org/10.1016/j.humpath.2015.07.015
[12] X. Huang, L. Hazlett, W. Du, & R. Barrett. Sigirr promotes resistance againstpseudomonas aeruginosakeratitis by down-regulating type-1 immunity and il-1r1 and tlr4 signaling, The Journal of Immunology, vol. 177, no. 1, p. 548-556, 2006. https://doi.org/10.4049/jimmunol.177.1.548
[13] M. Khan, T. Steiner, H. Sham, K. Bergstrom, J. Huang, K. Assi, et al. The single igg il-1–related receptor controls tlr responses in differentiated human intestinal epithelial cells, The Journal of Immunology, vol. 184, no. 5, p. 2305-2313, 2010. https://doi.org/10.4049/jimmunol.0900021
[14] Z. Wen, Y. Xia, Y. Zhang, Y. He, C. Niu, R. Wu, et al. Sigirr-caspase-8 signaling mediates endothelial apoptosis in kawasaki disease, Italian Journal of Pediatrics, vol. 49, no. 1, 2023. https://doi.org/10.1186/s13052-022-01401-8
[15] D. Blok, M. Lieshout, A. Hoogendijk, S. Florquin, O. Boer, C. Garlanda, et al. Single Immunoglobulin Interleukin-1 Receptor-Related Molecule Impairs Host Defense during Pneumonia and Sepsis Caused by Streptococcus Pneumoniae, Journal of Innate Immunity, vol. 6, no. 4, p. 542-552, 2014. https://doi.org/10.1159/000358239
c