[1] Mariotti F R, Supino D, Landolina N, et al. IL-1R8: A molecular brake of anti-tumor and anti-viral activity of NK cells and ILC[C]//Seminars in Immunology Academic Press, 2023, 66: 101712.
[2] Carty M, Kearney J, Shanahan K A, et al. Cell survival and cytokine release after inflammasome activation is regulated by the Toll-IL-1R protein SARM[J]. Immunity, 2019, 50(6): 1412-1424. e6.
[3] Martin M U, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family[J]. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2002, 1592(3): 265-280.
[4] Huang Xun. Experimental study of SIGIRR regulating HMGB1-induced inflammatory response of alveolar epithelial cells A549. Diss. Fourth Military Medical University, 2012.
[5] Thomassen E, Renshaw B R, Sims J E. Identification and characterization of SIGIRR, a molecule representing a novel subtype of the IL-1R superfamily[J]. Cytokine, 1999, 11(6): 389-399.
[6] Lech M, Skuginna V, Kulkarni O P, et al. Lack of SIGIRR/TIR8 aggravates hydrocarbon oil-induced lupus nephritis[J]. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland, 2010, 220(5): 596-607.
[7] Drexler S K, Kong P, Inglis J, et al. SIGIRR/TIR-8 is an inhibitor of toll-like receptor signaling in primary human cells and regulates inflammation in models of rheumatoid arthritis[J]. Arthritis & Rheumatism, 2010, 62(8): 2249-2261.
[8] Li L, Wei J, Li S, et al. The deubiquitinase USP13 stabilizes the anti-inflammatory receptor IL-1R8/Sigirr to suppress lung inflammation[J]. EBioMedicine, 2019, 45: 553-562.
[9] Li L, Wei J, Suber T L, et al. IL-37-induced activation of glycogen synthase kinase 3β promotes IL-1R8/Sigirr phosphorylation, internalization, and degradation in lung epithelial cells[J]. Journal of cellular physiology, 2021, 236(8): 5676-5685.
[10] Wang Q, Sun Z, Xia W, et al. Role of USP13 in physiology and diseases[J]. Frontiers in Molecular Biosciences, 2022, 9: 977122.
[11] Garlanda C, Anders H J, Mantovani A. TIR8/SIGIRR: an IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization[J]. Trends in immunology, 2009, 30(9): 439-446.
[12] Bertilaccio M T S, Simonetti G, Dagklis A, et al. Lack of TIR8/SIGIRR triggers progression of chronic lymphocytic leukemia in mouse models[J]. Blood, The Journal of the American Society of Hematology, 2011, 118(3): 660-669.
[13] Riva F, Bonavita E, Barbati E, et al. TIR8/SIGIRR is an interleukin-1 receptor/toll like receptor family member with regulatory functions in inflammation and immunity[J]. Frontiers in immunology, 2012, 3: 322.
[14] Heinig K, Sperandio M. Interleukin-1R8: balancing inflammation and hemostasis in platelets[J]. Cardiovascular Research, 2016, 111(4): 307-309.
[15] Molgora M, Barajon I, Mantovani A, et al. Regulatory role of IL-1R8 in immunity and disease[J]. Frontiers in immunology, 2016, 7: 149.
[16] Giannoudaki E, Stefanska A M, Lawler H, et al. SIGIRR negatively regulates IL-36-driven psoriasiform inflammation and neutrophil infiltration in the skin[J]. The Journal of Immunology, 2021, 207(2): 651-660.
[17] Costello D A, Carney D G, Lynch M A. α-TLR2 antibody attenuates the Aβ-mediated inflammatory response in microglia through enhanced expression of SIGIRR [J]. Brain, behavior, and immunity, 2015, 46: 70-79.
[18] Qin J, Qian Y, Yao J, et al. SIGIRR inhibits interleukin-1 receptor- and toll-like receptor 4-mediated signaling through different mechanisms[J]. Journal of Biological Chemistry, 2005, 280(26): 25233-25241.
[19] Zhang C, Wu X, Zhao Y, et al. SIGIRR inhibits toll-like receptor 4, 5, 9-mediated immune responses in human airway epithelial cells[J]. Molecular biology reports, 2011, 38: 601-609.
[20] Zhao R, Song C, Liu L, et al. Single immunoglobulin and Toll-interleukin-1 receptor domain containing molecule protects against severe acute pancreatitis in vitro by negatively regulating the Toll-like receptor-4 signaling pathway: A clinical and experimental study[J]. Molecular Medicine Reports, 2020, 22(4): 2851-2859.
[21] Gulen M F, Kang Z, Bulek K, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation[J]. Immunity, 2010, 32(1): 54-66.
[22] Jiang Keguo. The regulation of SIGIRR on the activation of NF-κB in human renal tubular epithelial cells, the feedback regulation of NF-κB on SIGIRR and the preliminary study on the mechanism of SIGIRR regulation of EMT. Diss. Anhui Medical University, 2015.
[23] Horne D J, Randhawa A K, Chau T T H, et al. Common polymorphisms in the PKP3-SIGIRR-TMEM16J gene region are associated with susceptibility to tuberculosis [J]. Journal of Infectious Diseases, 2012, 205(4): 586-594.
[24] Garlanda C, Di Liberto D, Vecchi A, et al. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling[J]. The Journal of Immunology, 2007, 179(5): 3119-3125.
[25] Costello D A, Watson M B, Cowley T R, et al. Interleukin-1α and HMGB1 mediate hippocampal dysfunction in SIGIRR-deficient mice[J]. Journal of Neuroscience, 2011, 31(10): 3871-3879.
[26] Martin M U. Special aspects of interleukin-33 and the IL-33 receptor complex[C]//Seminars in immunology. Academic Press, 2013, 25(6): 449-457.
[27] Lunding L, Webering S, Vock C, et al. IL-37 requires IL-18Rα and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice[J]. Allergy, 2015, 70(4): 366-373.
[28] Xiao H, Gulen M F, Qin J, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation and tumorigenesis[J]. Immunity, 2007, 26(4): 461-475.
[29] Liu J, Chen Y, Liu D, et al. Ectopic expression of SIGIRR in the colon ameliorates colitis in mice by downregulating TLR4/NF-κB overactivation[J]. Immunology letters, 2017, 183: 52-61.
[30] Che Y Y, Shi X, Zhong X D, et al. Resveratrol prevents liver damage in MCD-induced steatohepatitis mice by promoting SIGIRR gene transcription[J]. The Journal of Nutritional Biochemistry, 2020, 82: 108400.
[31] Molgora M, Bonavita E, Ponzetta A, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity[J]. Nature, 2017, 551(7678): 110-114.
[32] Bodaszewska-Lubas M, Liao Y, Zegar A, et al. Dominant-Negative Form of SIGIRR: SIGIRRΔE8 Promotes Tumor Growth Through Regulation of Metabolic Pathways[J]. Journal of Interferon & Cytokine Research, 2022, 42(9): 482-492.

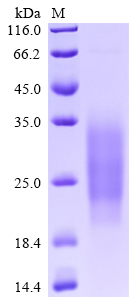
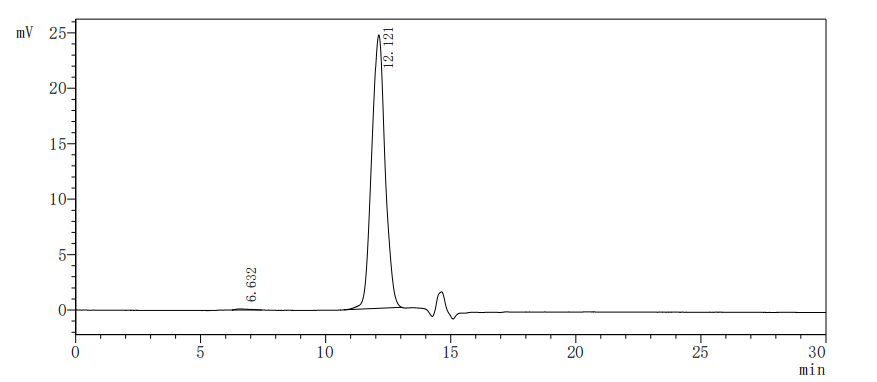



Comments
Leave a Comment