The Battle of Helicobacter Pylori
Helicobacter pylori (HP) are micro-aerobic bacteria that survive in each area of the stomach and duodenum. HP were discovered by Barry Marshall and Robin Warren, and because of this, they won the 2005 Nobel Prize in Physiology.
The proliferation of HP in the body can cause stomach pain, hyperchlorhydria, gastric ulcer and even stomach cancer when getting a chance. Since they cause so many troubles everywhere, if you get it unfortunately, you should kill it.
Since everyone hates HP, who is fighting for it? We search information around and found out that is SHP1 and SHP2. What are the details?
Currently, stomach cancer is the second cancer killer of mankind, especially in East Asia, which results in the death of about 50,000 people each year in Japan. Most of stomach cancer cases are caused by HP infection. Studies have shown that after intruding into the stomach cells, cytotoxin-associated gene A protein (cagA protein) of HP binds to an enzymes called SHP2 (SH2-containing tyrosine phosphatase 2), eventually causing stomach cancer[1]. We can say that SHP2 is the carcinogenic accomplice of HP. However, what is SHP2?
SHP2 is a protein tyrosine phosphatase encoded by protein tyrosine phosphatase N11 (the PTPN11) gene, and its molecular structure consists of two Src homology (N-SH2 and C-SH2), a catalytic activity of protein tyrosine phosphatase domain, a C-terminal tail domain containing several tyrosine phosphorylation sites and a proline-rich motif[2]. Higashi et al.[1] found that SHP2 specifically binds to tyrosine phosphorylated cagA (cagA is the primary causative factor of HP), then activates RAS signaling pathway, and initiates extracellular signal transduction pathway. Thus, SHP2-mediated phosphorylation is considered as one of the mechanisms of the occurrence of stomach cancer caused by cagA-positive HP. Namely, SHP2 helps HP settle in the human stomach cells.
In March this year, a Japanese research team led by Professor Hatakeyama Chang published a paper in the journal of Nature Microbiology. The paper has shown that an enzyme called SHP1 can help fight against stomach cancer caused by HP[3]. So, what is SHP1? And what is the relationship between SHP1 and SHP2?
The true features of SHP1
Protein tyrosine phosphatase 1 (SHP1), also named phospho-tyrosine phosphatase 1C (PTP1C), hematopoietic cell phosphatase (HCP) and non-receptor protein tyrosine phosphatase (PTPN6), is one member of protein tyrosine phosphatase family containing SH2 domain[4]. In the early 1990s, SHP1 has been isolated and purified by several researcher groups at the University of Washington, and its cDNA was also cloned successfully. Now it is known that the SHP1 gene is located on human chromosome 12p13 and the protein SHP1 expressed by this gene consists of a N-terminal containing two-tandem SH2 domain (N-SH2 and C-SH2), a single central catalytic active region and a C-terminal containing multiple phosphorylation sites in the tail. The activity regulation of SHP1 is important for its function. Normally, the activity of SHP1 is suppressed by the binding of SH2 N-terminal domain to the central catalytic activity region at the molecular level; while the binding of peptides containing phosphorylated tyrosyl to SH2 N-terminal domain leads to their gradual dissociation, therefore SHP1 is activated[5].
The relationship between SHP1 and SHP2
SHP1 and SHP2 have similar names, so what is their relationship? Their role in HP-induced stomach cancer is opposite, and what causes this?
SHP-2 and SHP-1 have the similar molecular structure, and their PTP domains have 60% homology. They both contain two SH2 domains in the N-terminal with phosphotyrosine independent binding sites and a phosphatase region in the C-terminal (FIG homology following more intuitive). Although SHP-2 and SHP-1 demonstrate a high homology and can be expressed in the immune system and hematopoietic system, they have distinct functions and cannot replace each other, because the substrates identified by their SH2 domains are different and their catalytic domains also have some difference.
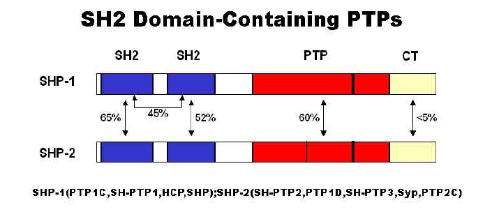
Image cited from "The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury."
The inhibition of stomach cancer by SHP1
When cagA protein, the main virulence factors of HP, binds to SHP1, its oncogenic activity is neutralized; when it binds to SHP2, its carcinogenic activity is stimulated. It means that after HP infection, the extent to which these two enzymes functions respectively will decide whether the person will get stomach cancer or not.
Although we have known the enzyme SHP1 can inhibit stomach cancer caused by HP, many questions still remain to be answered.
For example:
1. The mechanism by which SHP1 inhibits stomach cancer is unclear.
2. SHP1 enhancer drugs need to be developed.
3. Whether SHP1 enhancer will result in human metabolic disorders is unclear.
4. What is the competitive regulatory mechanism of the "battle" between SHP1 and SHP2.
......and many more.
Cusabio, as a good partner of life science research, manufactures and provides SHP1 and SHP2 related products.
1. Rabbit anti-Human PTPN6 Polyclonal antibody (product code: CSB-PA019043HA01HU)
2. Recombinant Human Tyrosine-protein phosphatase non-receptor type 6(PTPN6) (product code: CSB-YP019043HU)
3. Recombinant Rat Tyrosine-protein phosphatase non-receptor type 6(Ptpn6) (product code: CSB-YP019043RA)
4. Recombinant Mouse Tyrosine-protein phosphatase non-receptor type 6(Ptpn6) (product code: CSB-YP019043MO)
Reference:
[1] Higashi H, Nakaya A, Tsutsumi R, et al. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation [J]. J Biol Chem, 2004, 279(17):17205-17216.
[2] Poole AW, Jones ML. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail [J]. Cell Signal, 2005, 17(11):1323-1332.
[3] Priya S, Naoko MK, Takeru H. Host SHP1 phosphatase antagonize Helicobacter pylori CagA and can be downregulated by Epstein-Barr virus. Nature Microbiology March 2016.
[4] Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels [J]. Immunol Rev, 2009, 228:342-359.
[5] Wu C, Sun M, Liu L, et al. The function of the protein tyrosine phosphatase SHP-1 in cancer [J]. Gene, 2003, 306:1-12.
Cite this article
CUSABIO team. The Battle of Helicobacter Pylori. https://www.cusabio.com/c-19696.html





Comments
Leave a Comment