The high mortality of cancer is mainly due to the metastatic growth of cancer cells. Cancer metastasis initiates when cellular programs that enable dissemination and
seeding in the distant organs are transiently activated. Genetic, transcriptional, and translational heterogeneity has been proven to be essential to this dynamic process. However, the role of metabolic
heterogeneity in metastatic growth is poorly understood.
Recently, Matteo Rossi et al. demonstrated that metabolic heterogeneity is associated with cancer metastasis. They found that the loss of
phosphoglycerate dehydrogenase (PHGDH) enhances cancer dissemination and metastasis formation [1]. Lack of PHGDH protein expression activates the hexosamine–sialic acid pathway,
which provides precursors for protein glycosylation. The occurrence of abnormal protein glycosylation, including elevated sialylation of integrin αvβ3, potentiates cell migration and
invasion. Suppression of sialylation neutralizes the pro-metastatic effect of cancer cells containing low PHGDH.
PHGDH-related Theory and Research
PHGDH is widely expressed in all organisms and has more than three basic structural forms: type I, II, and III. The human PHGDH belongs to type I. All three types of PHGDH
consist of two common domains: the structural-binding domain and the cofactor-binding domain. Type I PHGDH also contains the aspartate kinase-chorismate mutase-tyrA prephenate dehydrogenase (ACT)
domain that acts as a binding site for serine to give feedback repression and the allosteric substrate binding (ASB) domain.
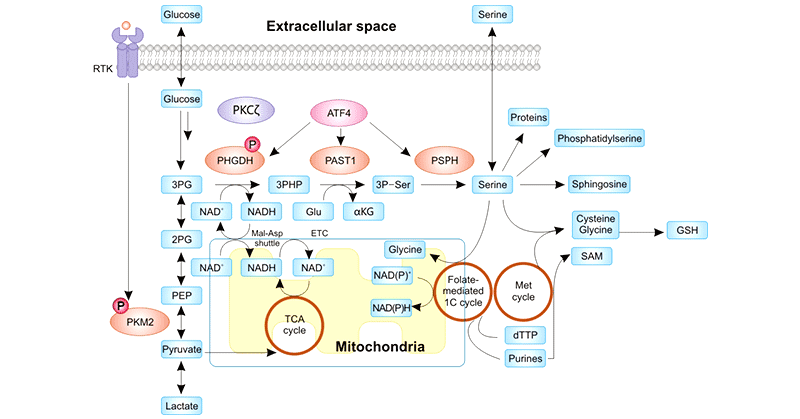
PHGDH, the first rate-limiting enzyme in the serine biosynthesis pathway, catalyzes the glycolytic intermediate 3-phosphoglycerate into phospho-hydroxypyruvate, which is
eventually turned into serine via transamination (PSAT1) and phosphate ester hydrolysis (PSPH) reactions [2]. serine is metabolized and incorporated into various biomolecules.
Figure 1. Regulation of the de novo serine synthesis pathway and the myriad fates of the serine product
This picture is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7092752/
Serine is an important non-essential amino acid that has multiple uses for cancer cells, including nucleotides for genome replication and ribosomal RNA, lipids for
membranes, amino acids for proteins, and other cellular building blocks [3]. Serine metabolism not only provides essential precursors for the synthesis of proteins, nucleic acids, and lipids in
tumor cells but also fuels the maintenance of REDOX homeostasis in tumor cells. Therefore, serine metabolism is essential for tumor growth. Elevated serine biosynthesis is one of many metabolic
alterations described in cancer cells.
As an essential enzyme for serine biosynthesis, PHGDH is considered a nutritious partner of cancer cells. PHGDH overexpression has been found in multiple types of cancer,
including breast cancer, melanoma, nasopharyngeal carcinoma, glioma, hepatocellular carcinoma, and small intestinal carcinoma. PHGDH knockdown leads to reduced cancer cell growth.
Richard Possemato et al. found that the elevated PHGDH caused an increase in the metabolic flux through the serine synthesis pathway, which in turn significantly
contributed to the influx of glutamate through the tricarboxylic acid cycle-α-ketoglutarate pathway [4]. Inhibition of the expression of PHGDH led to the reduction of serine synthesis in
breast cancer cells and subsequent suppression of cancer cell growth.
A 2016 report by Pacold ME et al. revealed that two PHGDH inhibitors NCT-502 and NCT-503 could selectively stop the growth of PHGDH-dependent cancer cells
[5]. Besides inhibiting the synthesis of serine, PHGDH inhibitors did not disrupt other amino acids except aspartic acid. The researchers also found that these two inhibitors not only affected the
glucose-derived serine synthesis pathway but also reduced the incorporation of endogenous and exogenous serine into nucleotides of one-carbon units. In short, PHGDH inhibitors cut off the nutritious
supply for cancer cells, forcing them to starve to death.
Weiwei Yang et al. found that mono-ubiquitination modification of PHGDH promoted colorectal cancer (CRC) metastasis [6]. Cul4A-DDB1-mediated
mono-ubiquitination of PHGDH at K146 promoted the interaction between PHGDH and molecular chaperone DNAJA1, thus promoting the formation of PHGDH tetramer and upregulating PHGDH activity. The
increase of PHGDH activity increased the expression of its downstream metabolite S-adenosylmethionine (SAM) in cells. High levels of SAM selectively activated methyltransferase SETD1A and promoted
the trimethylation of H3K4me3 modification of LAMC2 and CYR61 promoters, thereby facilitating the expression of LAMC2 and CYR61 and the metastasis of CRC cells to the liver.
Metabolic rewiring is considered a hallmark of cancer cells. Daniela Annibali and Sarah-Maria Fendt found that PHGDH translocated from the cytosol to the nucleus after
nutrient stress [7]. Nuclear PHGDH decreased local NAD+ availability necessary for the PARylation of the transcription factor c-JUN, leading to the reduction of the c-JUN activity, thus
contributing to continuous cancer cell proliferation. Chunmin Ma et al. also confirmed a similar view through the observation of glucose restriction-induced PHGDH transition from the cytosol into the
nucleus [8]. They noted the functional importance of alternative PHGDH activity in tumorigenesis.
PHGDH Proteins and Antibodies
CUSABIO provides a variety of PHGDH products for research use, including recombinant PHGDH proteins and anti-PHGDH antibodies.
References
[1] Rossi, M., Altea-Manzano, P., Demicco, M. et al. PHGDH heterogeneity potentiates cancer cell dissemination and metastasis [J]. Nature 605, 747–753 (2022).
[2] Snell K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues [J]. Adv Enzyme Regul. 1984;22:325–400.
[3] Locasale J.W. 2013. Serine, glycine and one-carbon units: Cancer metabolism in full circle [J]. Nat. Rev. Cancer. 13:572–583.
[4] Possemato R, Marks KM, Shaul YD, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer [J]. Nature. 2011 Aug 18;476(7360):346-50.
[5] Pacold ME, Brimacombe KR, Chan SH, et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate [J]. Nat Chem Biol. 2016 Jun;12(6):452-8.
[6] Yajuan Zhang,1 Hua Yu, et al. (2021). Cul4A-DDB1–mediated monoubiquitination of phosphoglycerate dehydrogenase promotes colorectal cancer metastasis via increased S-
adenosylmethionine [J]. The Journal of Clinical Investigation, 131(21).
[7] Daniela Annibali and Sarah-Maria Fendt. Nuclear PHGDH protects cancer cells from nutrient stress [J]. Nature Metabolism volume 3, pages1284–1285 (2021).
[8] Chunmin Ma, Ke Zheng, et al. The alternative activity of nuclear PHGDH contributes to tumour growth under nutrient stress [J]. Nat Metab. 2021 Oct;3(10):1357-1371.
CUSABIO team. PHGDH - An Enzyme Related to Cancer Progression and Metastasis. https://www.cusabio.com/c-19670.html



Comments
Leave a Comment