Apoptosis mediated by endoplasmic reticulum
1. Apoptosis instruction
2. Pathways
2.1 Endogenous mitochondrial
pathway
2.2 Endogenous endoplasmic
reticulum pathway
Endoplasmic
reticulum(ER) is the main site of protein processing, as well a major storage compartment
of Ca2+ in cell, therefore, being decisively important in the
synthesis, folding, modification and transport of protein, and maintaining Ca2+ homeostasis.
The accumulation of unfold or misfolded proteins and disturbance of Ca2+
homeostasis in endoplasmic
reticulum trigger the ER stress response (ERSR), which
facilitates protein folding and removal of damaged proteins, and stabilizes ER
Ca2+ homeostasis, but excessive ER stress response triggers apoptotic signals
and induces apoptosis.
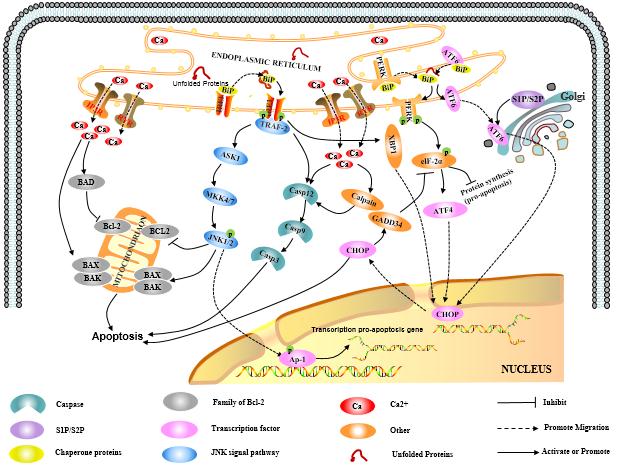
2.2.1 Regulation of apoptosis by the misfolded
protein response
In eukaryotic cells, the unfolded protein response (UPR) is a self-protective mechanisms that responds to ER stress. The highly intense and
prolonged UPR triggers three transmembrane protein PERK,
IREI and ATF6 to repair cells, and samutalneously
induces three ER
stress-mediated apoptosis pathways.
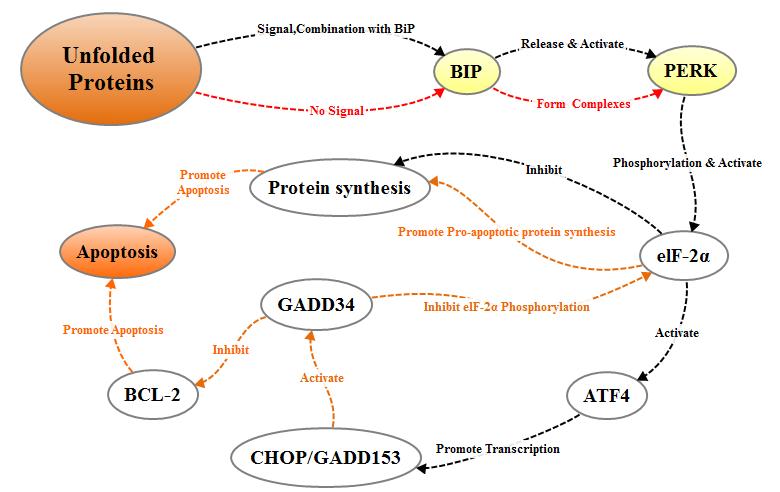
2.2.1.1 PERK pathway
PERK is a transmembrane protein kinase of the PEK family resident in the endoplasmic
reticulum (ER) membran. When proteins are properly folded, PERK is in
combination with formolecular chaperone like BiP/GRP78 to form stable compound,
the combination of misfolded proteins and BiP/GRP78 will interfere with the
interaction between PERK and BiP/GRP78. The released PERK is activated by
oligomerization and reverse autophosphorylation, and activated PERK
phosphorylates the alpha subunit of translation initiation factor 2 (eIF-2a).
In the early stage of stress response, phosphorylated eIF2α inhibits the
translation and synthesis of proteins and reduces the load of protein folding
in the endoplasmic reticulum, thereby protecting the cells. With the increase
of the intensity and duration of stress reaction, phosphorylated eIF-2α induces
the transcriptional expression of transcription factor ATF4, and ATF4 can promote
the expression of apoptotic signal molecule CHOP / GADD153, which in turn tiggers
cell apoptosis.
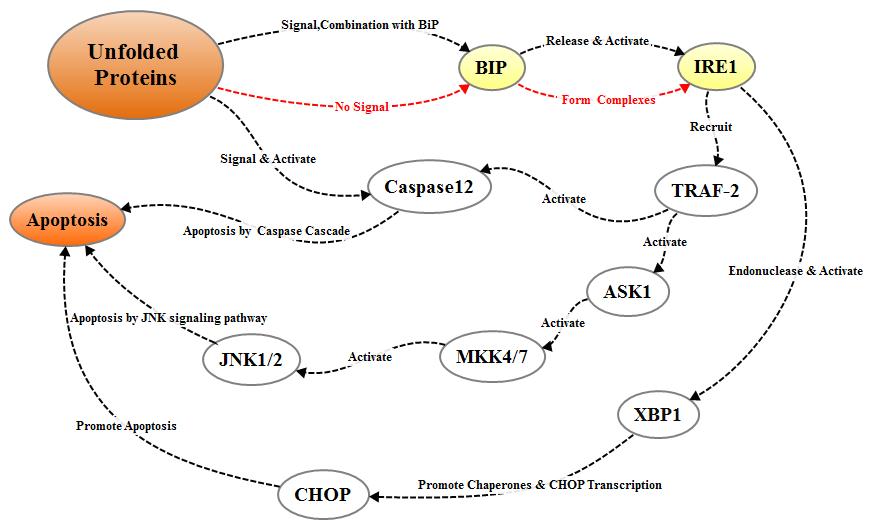
2.2.1.2 IREI pathway
IREI is another protein kinase
located on the endoplasmic reticulum membrane. The IREI signal pathway is
activated in the same way as PERK. When unfolded proteins accumulate in the
endoplasmic reticulum, the IREI-BIP / GRP78 complex dissociates, the released
IREI becomes oligomerized and activated after reverse autophosphorylation; The
activated IREI can transmit cell survival signal and apoptosis signal. During
the process of apoptosis, the activated IRE1 recruits cytosolic regulatory
protein TRAF-2, indirectly recruiting and activating c-Jun N-terminal kinase, which
inhibits apoptosis inhibitor proteins of the Bcl-2 family through
phosphorylation. On the other hand, activated TRAF-2 simultaneously activates
Caspase12 and initiates caspase cascade to mediate apoptosis. In addition, IRE1
also has ribonuclease activity which cleaves XBP1 mRNA to promotes the
maturation of XBP1 mRNA and enhances the transcriptional expression of
molecular chaperone protein and CHOP / GADD153, thereby promoting apoptosis.
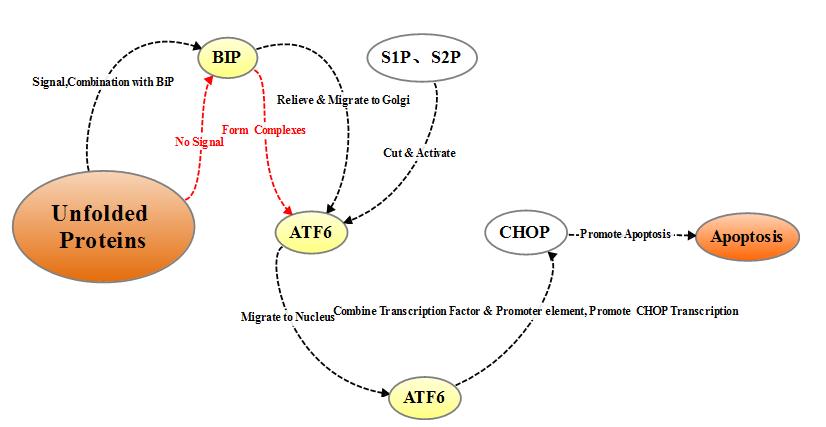
2.2.1.3 ATF6 pathway
ATF6
is a type II transmembrane protein located on the endoplasmic reticulum. The
N-terminal intracellular domain of ATF6 contains the DNA transcriptional
activation domain and nuclear localization signal of b-ZIP. Under non-stress
conditions, ATF6 resides in the endoplasmic reticulum membrane in the form of
zymogen, and under endoplasmic reticulum stress, ATF6 is transported to the
Golgi apparatus via vesicles, where it undergoes cleavage by site-1 and site-2
(S1P and S2P) proteases and then relocate to the nucleus with the nuclear
localization signal, inducing transcriptional expression of the endoplasmic
reticulum stress gene including CHOP / GADD153 in the nucleus.
Both
PERK, IRE1 and ATF6 signal pathways can induce the expression of CHOP/GADD153,
as a direct result of of endoplasmic reticulum stress. CHOP/GADD153 plays an
important role in cell growth arrest and cell death.
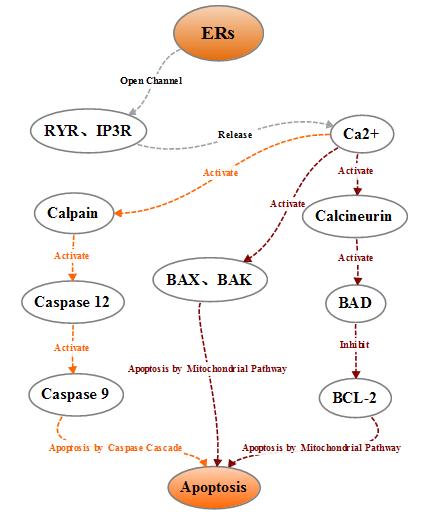
2.2.2 Regulation of apoptosis by the imbalance of Ca2+ homeostasis
During
normal operation of the cells, endoplasmic reticulum releases Ca2 + in the
endoplasmic reticulum into the cytoplasm mainly through the RyR and IP3R
channels, and pumps intracellular Ca2 + into the endoplasmic reticulum lumen
through a calcium pump to maintain the endoplasmic reticulum Ca2 + homeostasis.
When the endoplasmic reticulum receives the stress signal, the Ca2 +
homeostasis in the endoplasmic reticulum is broken, a large amount of Ca2 +
enters the intracellular and mitochondria, which on the one hand influences the
activity of mitochondria and Bcl-2 family proteins and leads the cells to
apoptosis, on the other hand activates the intracellular neutral cysteine
endopeptidase Calpain, the activated Calpain can activate caspase cascade and
affect apoptosis.
Click for more apoptotic antibodies
Past review
Apoptosisi mediated by mitochondria
The next notice
Apoptosisi mediated by death receptor
3. Cited References
[1] Green D R, Kroemer
G. The pathophysiology of mitochondrial cell death [J]. Science, 2004, 305:
626-629. Groenendyk J, Michalak M. Endoplasmic reticulum quality control and
apoptosis [J]. Acta Biochimica Polonica, 2005, 52(2): 381-395.
[2] Bastida-Ruiz D,
Aguilar E, Ditisheim A, et al. Endoplasmic reticulum stress responses in
placentation - A true balancing act [J]. Placenta, 2017, 57: 163-169.
[3] Kang-sheng LIU, Zheng-hang
PENG, Weng-jun CHENG, et al. Endoplasmic reticulum stress-induced apoptosis in
the development of reproduction [J]. Reproductive and Developmental Medicine,
2016, 27(1): 51-59.
[4] Li J, Lee B, Lee A
S. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and
activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53
[J]. Journal of Biological Chemistry, 2006, 281(11): 7260-7270.
[5] Burton G J, Yung H
W, Murray A J. Mitochondrial - Endoplasmic reticulum interactions in the trophoblast:
Stress and senescence [J]. Placenta, 2017, 52: 146-155.
[6] Marchi S, Patergnani
S, Missiroli S, et al. Mitochondrial and endoplasmic reticulum calcium
homeostasis and cell death [J]. Cell Calcium, 2017.
[7] Breckenridge D G,
Germain M, Mathai J P, et al. Regulation of apoptosis by endoplasmic reticulum
pathways [J]. Oncogene, 2003, 22(53): 8608-8618.
Cite this article
CUSABIO team. Apoptosis mediated by endoplasmic reticulum. https://www.cusabio.com/c-20466.html









Comments
Leave a Comment