Apoptosis mediated by mitochondria
1. Apoptosis instruction
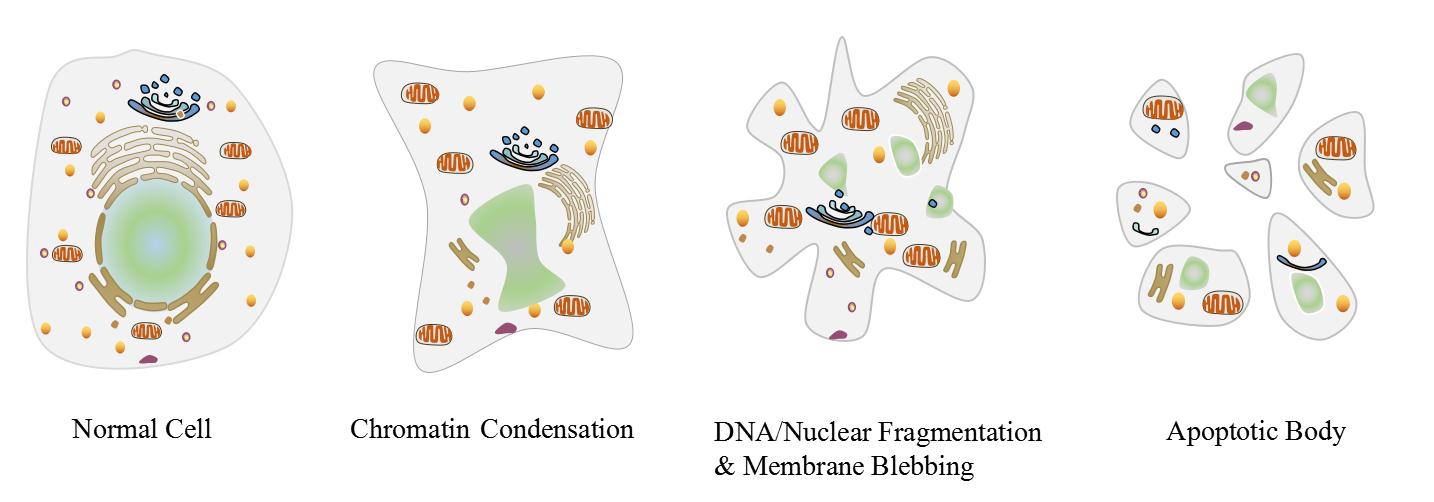
Apoptosis is a genetically determined process of cell self-destruction to maintain its own internal environment under physiological or pathological conditions, while it is accompanied by a series of morphological and biochemical changes, including pyknosis, DNA fragmentation, cell membrane remodelling&blebbing, cell shrinkage, formation of apoptotic bodies and so on. Lastly, apoptotic cells are swallowed up by macrophages and die. Apoptosis is a normal death process of cell involving gene activation, expression and regulation, which is intent to adapt to its internal and external environment. In whole process of apoptosis, the plasma membrane still remain intact and does not cause any leakage of cytoplasm or inflammatory response.
2. Pathways
Apoptosis pathways fall into endogenous
mitochondrial pathway, endogenous endoplasmic reticulum pathway and
exogenous death receptor pathway. Meanwhile, apoptosis process also can be
mediated by granzyme B under certain conditions.
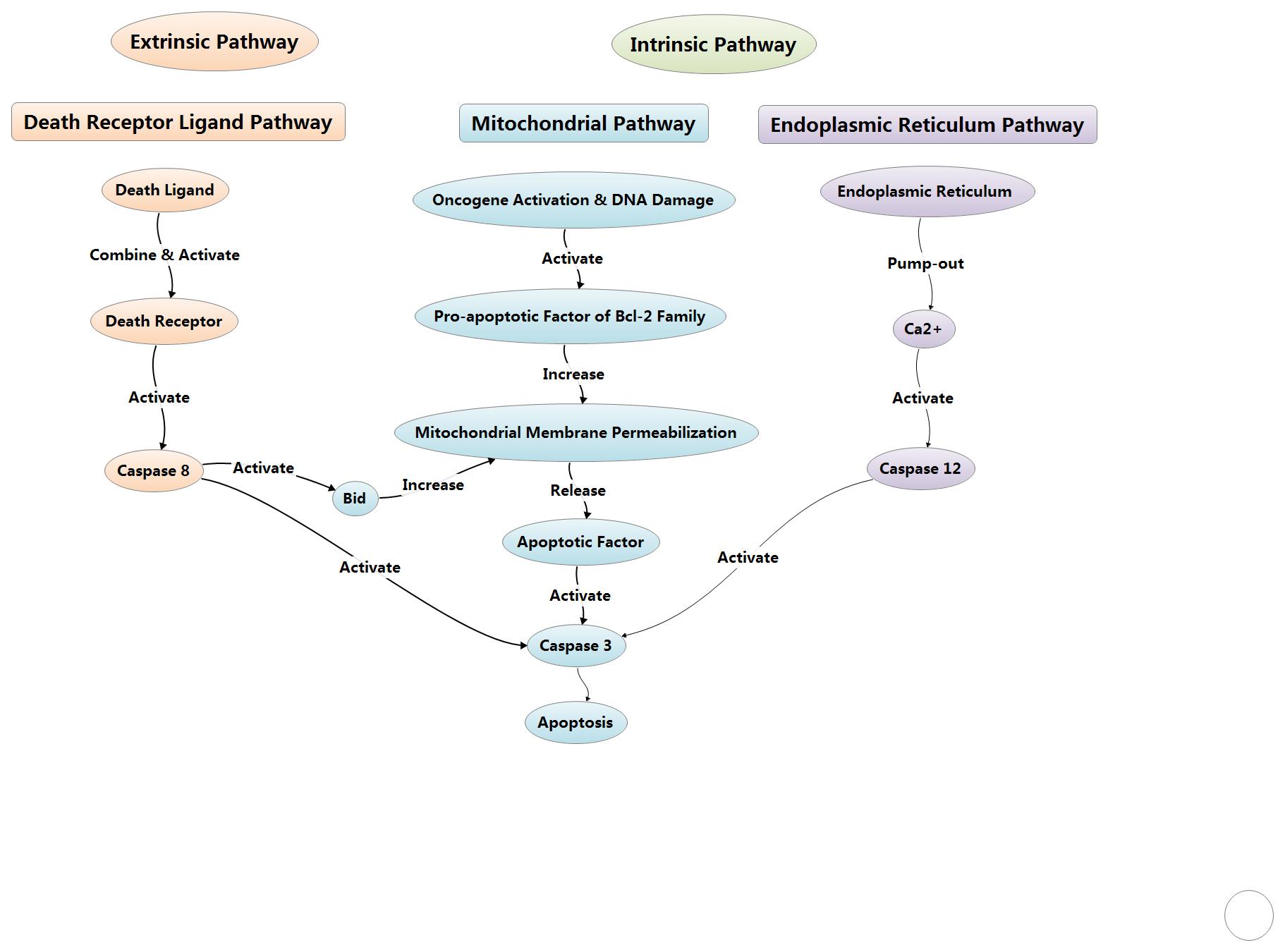
2.1 Endogenous mitochondrial pathway
The endogenous mitochondrial pathway in cells can be activated by
death ligand or when cells are suffering from apoptotic stimulating factors to
cause the death of cells. The stimulating factors include oncogene activation,
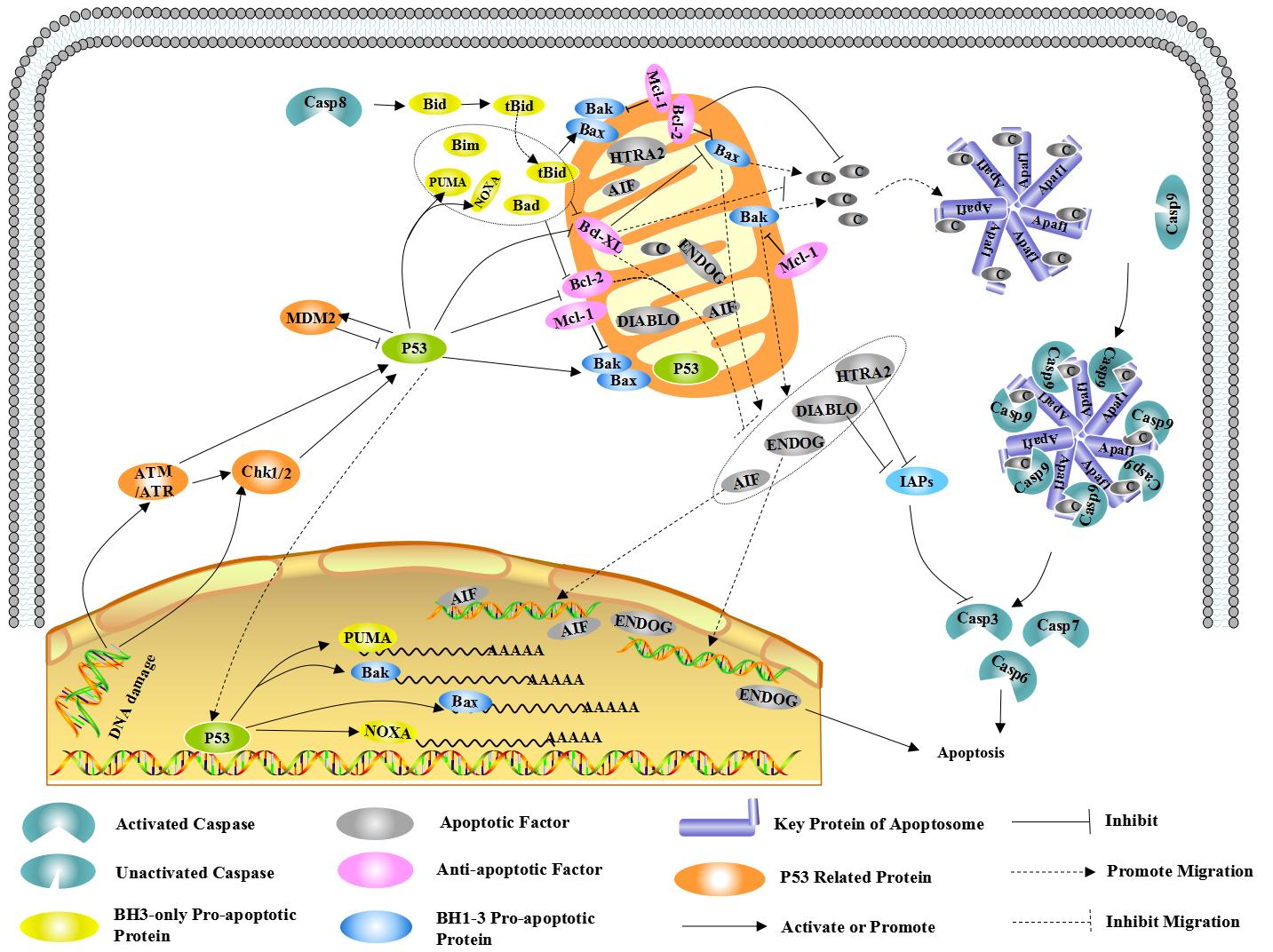
DNA damage, cell hypoxia, cell growth factor deletion, and so on. As for this pathway, Bcl-2 family could control the mitochondrial
outer membrane permeabilization via adjusting membrane potential.
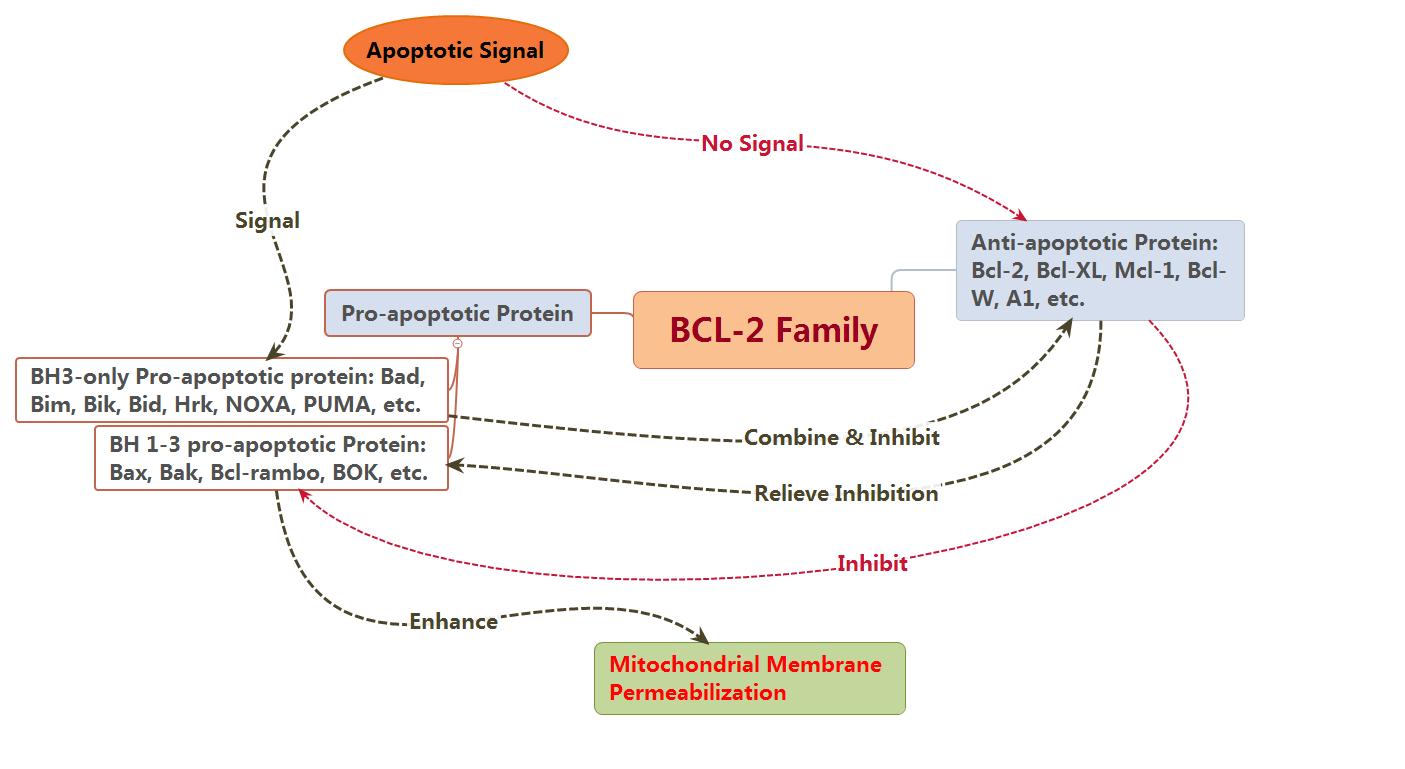
2.1.1 Bcl-2 family
BCL-2 family proteins are the regulators of apoptosis, which
consists of anti-apoptotic and pro-apoptotic members, and could control
apoptosis by governing mitochondrial outer membrane permeabilization (MOMP).
The pro-apoptotic protein can be divided into BH1-3 and BH3. The member of
pro-apoptotic protein, such as Bak, together with other members of
anti-apoptotic protein, such as Bcl-2 and Bcl-xL, all of them are mainly found
in mitochondrial membrane. Other members such as Bid and Bad are mainly found
in cytoplasm. Usually, Bax is mainly found in cytoplasm, when receiving
apoptotic signal, which could relocating on the surface of mitochondria and
form transmembrane pore to reduce membrane potential and increase membrane
permeability as well, finally, cause the release of apoptosis factor.
At present, there are two hypotheses for the
activated ways of Bax and Bak: direct and indirect modes.
Indirect mode: usually, the activity of Bax
and Bak is inhibited by anti-apoptotic protein, so which could be activated
in-directly if the anti-apoptotic activity has been inhibited by the members of
BH-3only family after receiving apoptotic signal.
Direct mode: BH3-only can be divided into activated protein and
kinase protein. The activated protein which have not received apoptotic signal
could combine with anti-apoptotic protein to inhibit the activation of Bax and
Bak. In contrast, both Bax and Bak could be activated directly if kinase
protein combines with anti-apoptotic protein to release activated protein as
soon as receiving apoptotic signal.
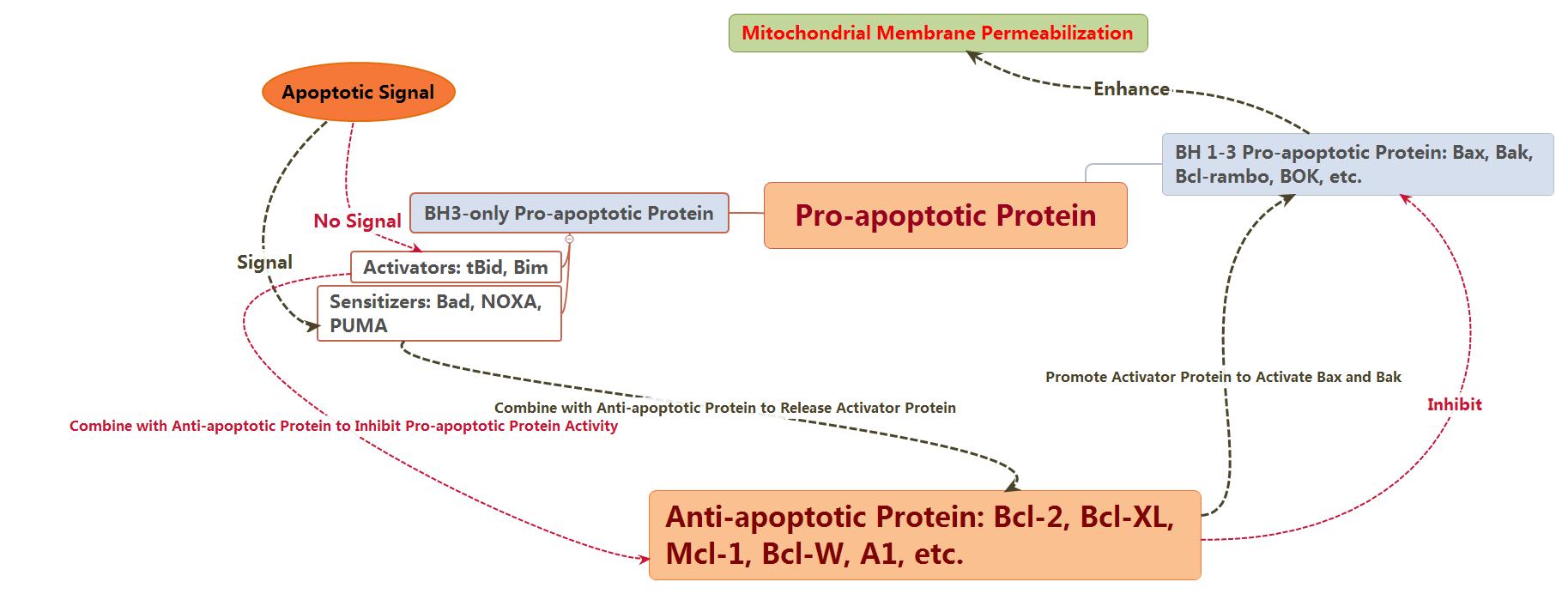
In conclusion, Bcl-2 family comprises anti-apoptotic and
pro-apoptotic members, which interacts with each other and determines together
whether the cells go into apoptosis process or not.
2.2.1 Apoptosis process mediated by mitochondria
The mitochondrial membrane potential reduction and membrane
permeability increasing could cause the release of endogenous mitochondrial
apoptotic factors, including CytC, AIF, SMAC/DIABLO, HTRA2/OMI and ENDOG. Cyt C
interacts with Apaf-1 after releasing into cells, to form apoptosis complex
with the help of ATP and dATP. Then apoptosis complex combines with
Pro-Caspase9 as well as activates it to form Caspase9. Next, Caspase9 could
cause a further activation for both Caspase3 and Caspase7 to start Caspase
cascade reaction and cut more than 100 kinds of substrate in cells, such asα-tubulin,
Actin, PARPA, Lamin, etc. Finally, the series of reactions cause apoptosis.
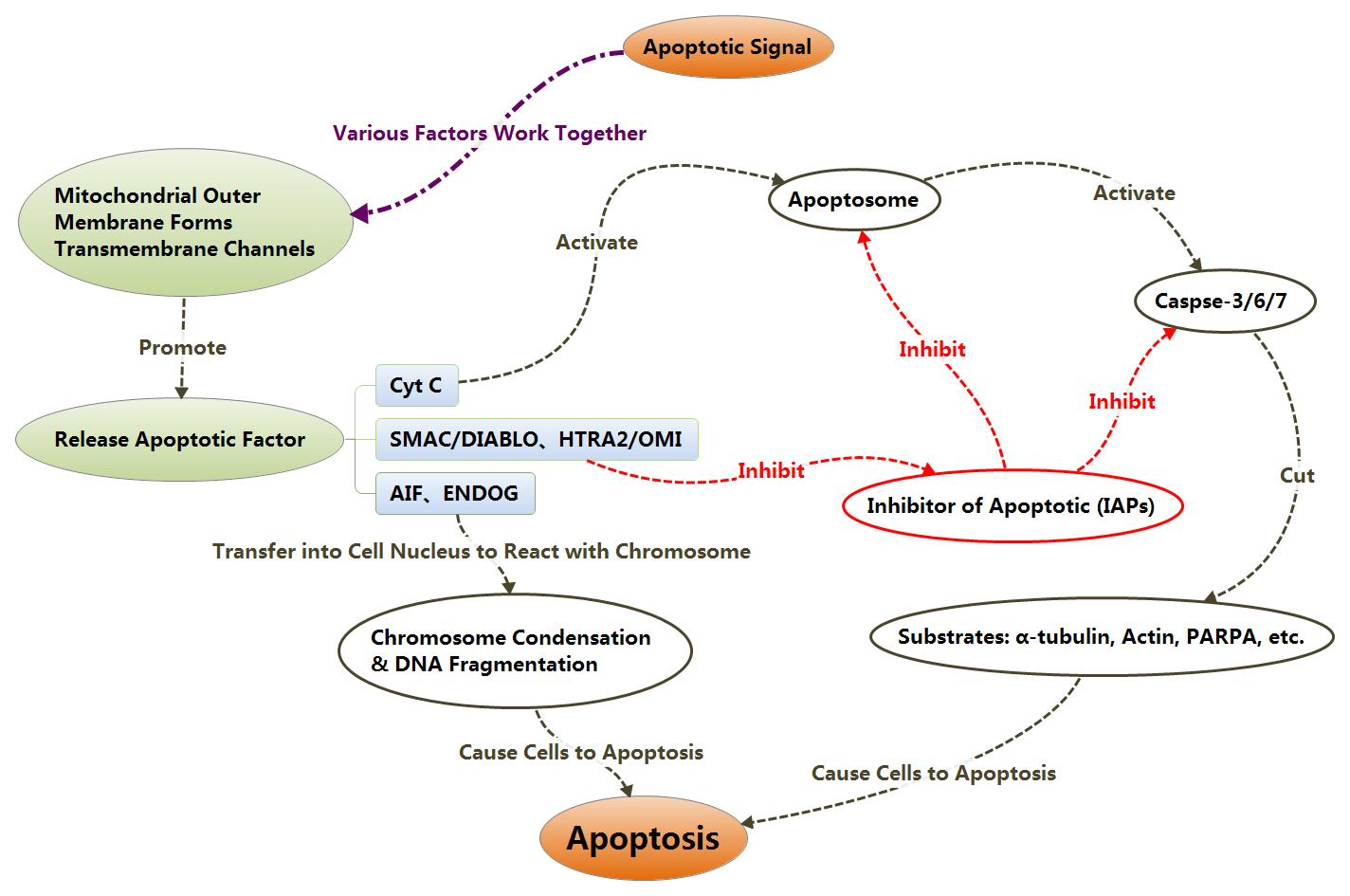
IAPs could inhibit the activation of Caspase3 and Caspase7 to
inhibit cell apoptosis. After releasing themselves from
mitochondrion into cytoplasm, SMAC/DIABLO and HTRA2/OMI could combine with IAPs
to relieve the inhibition of apoptosis from IAPs, thereby, promote apoptosis
indirectly.
With the change of mitochondrial membrane potential, AIF and ENDOG
also could be released into cytoplasm, then transferred into cell nucleus to
trigger chromosome condensation and DNA fragmentation as well, finally, causing
apoptosis.
Apoptosis
Mediated by Mitochondria
Click for more apoptotic antibodies
The next notice
Endogenous endoplasmic
reticulum pathway
3. Cited references
[1] Brenner D, Mak T W. Mitochondrial cell death
effectors [J]. Current Opinion in Cell Biology, 2009, 21: 871-877.
[2] Chalah A, Khosravi-Far R. The mitochondrial death
pathway [J]. Advances in Experimental Medicine and Biology, 2008, 615: 25-45.
[3] Lindsay J, Esposti M D, Gilmore A P. Bcl-2
proteins and mitochondria—Specificity in membrane targeting for death [J].
Biochimica et Biophysica Acta, 2011, 1813: 532-539.
[4] Ola M S, Nawaz M, Ahsan H. Role of Bcl-2 family
proteins and caspases in the regulation of apoptosis.[J]. Molecular and
Cellular Biochemistry, 2011, 351: 41-58.
[5] Pradelli L A, Bénéteau M, Ricci J E.
Mitochondrial control of caspase-dependent and -independent cell death [J].
Cellular and Molecular Life Sciences, 2010, 67: 1589-1597.
[6] Rong Y, Distelhorst C W. Bcl-2 protein family
members: versatile regulators of calcium signaling in cell survival and
apoptosis [J]. Annual Review of Physiology, 2008, 70: 73-91.
[7] Speidel D. Transcription-independent p53
apoptosis: an alternative route to death [J]. Trends in Cell Biology, 2010, 20:
14-24.
[8] Suen D F, Norris K L, Youle R J. Mitochondrial
dynamics and apoptosis [J]. Genes & Development, 2008, 22: 1577-1590.
[9] Danial N N, Korsmeyer S J. Cell Death: Critical
Control Points [J]. Cell, 2004, 116: 205-219.
[10] Green D R, Kroemer G. The pathophysiology of
mitochondrial cell death [J]. Science, 2004, 305: 626-629.
Cite this article
CUSABIO team. Apoptosis mediated by mitochondria . https://www.cusabio.com/c-20458.html










Comments
Leave a Comment