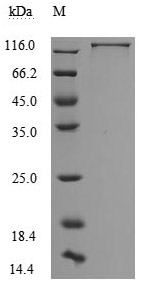
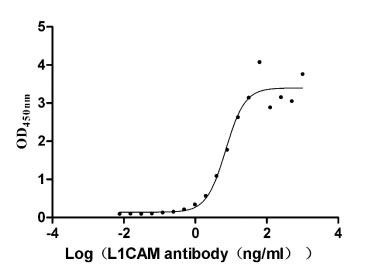
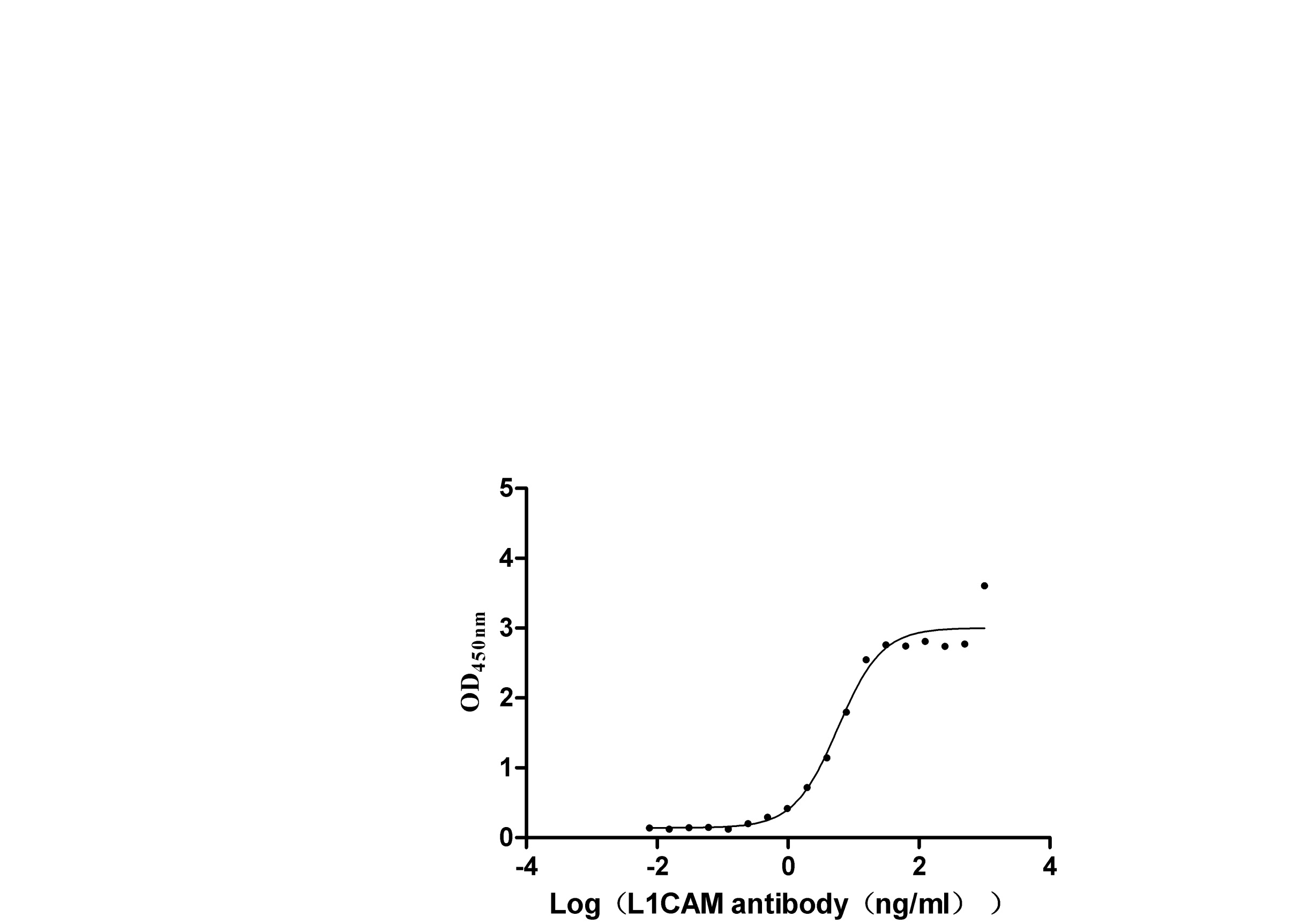
The recombinant human L1CAM protein, covering amino acids 20-1120, is generated using mammalian cell expression of a plasmid with an N-terminal 10xHis-tag gene sequence and C-terminal Myc-tag gene. This plasmid is constructed by cloning the gene fragment that encodes the 20-1120aa of human L1CAM. This L1CAM protein's purity exceeds 95% as measured by SDS-PAGE, and the LAL assay determines its endotoxin levels below 1.0 EU/μg. The L1CAM protein's functional activity is validated by ELISA, binding specifically to the L1CAM rabbit monoclonal antibody (CSB-RA595071A0HU), with an EC50 range of 5.384 to 9.380 ng/mL.
Human L1CAM is a significant transmembrane glycoprotein belonging to the immunoglobulin superfamily, primarily recognized for its critical roles in neuronal development and its implications in various cancers. Structurally, L1CAM is characterized by six immunoglobulin-like domains and five fibronectin type III repeats, which facilitate both homophilic and heterophilic interactions with other proteins, including integrins and various extracellular matrix components [1]. These interactions are essential for processes such as neurite outgrowth, neuronal migration, and axon fasciculation [2].
In the context of cancer, L1CAM is involved in tumor progression and metastasis. Its overexpression is frequently associated with poor prognosis across multiple cancer types, including ovarian, gastric, and colorectal cancers [3][4]. Studies have demonstrated that L1CAM enhances cell proliferation, migration, and invasion, thereby promoting aggressive tumor behavior [3][5]. The mechanism underlying this phenomenon involves the activation of signaling pathways, such as the PI3K pathway, which is crucial for cell survival and motility [3]. Furthermore, L1CAM's role in epithelial-mesenchymal transition (EMT) highlights its contribution to the invasive characteristics of tumors [6].
Research has indicated that targeting L1CAM with specific antibodies can significantly reduce metastasis in preclinical models, suggesting its utility in anti-cancer therapies [5]. Additionally, the presence of L1CAM in various tumor microenvironments has been linked to enhanced tumor cell survival and increased metastatic potential, making it a focal point for developing novel therapeutic strategies [4]. Moreover, L1CAM mutations have been implicated in genetic disorders, particularly in the context of L1 syndrome, which is characterized by neurological deficits and structural brain anomalies [7][8]. These mutations can disrupt the normal function of L1CAM, leading to significant developmental issues.
References:
[1] H. Kiefel, S. Bondong, N. Erbe-Hoffmann, J. Hazin, S. Riedle, J. Wolf, et al. L1cam–integrin interaction induces constitutive nf-κb activation in pancreatic adenocarcinoma cells by enhancing il-1β expression, Oncogene, vol. 29, no. 34, p. 4766-4778, 2010. https://doi.org/10.1038/onc.2010.230
[2] G. Moya, R. Michaelis, L. Holloway, & J. Sánchez. Prenatal diagnosis of l1 cell adhesion molecule mutations, Fetal Diagnosis and Therapy, vol. 17, no. 2, p. 115-119, 2002. https://doi.org/10.1159/000048020
[3] D. Chen, Z. Zeng, J. Yang, C. Ren, D. Wang, W. Wu, et al. L1cam promotes tumor progression and metastasis and is an independent unfavorable prognostic factor in gastric cancer, Journal of Hematology & Oncology, vol. 6, no. 1, 2013. https://doi.org/10.1186/1756-8722-6-43
[4] D. Cave, X. Hernando‐Momblona, M. Sevillano, G. Minchiotti, & E. Lonardo. Nodal-induced l1cam/cxcr4 subpopulation sustains tumor growth and metastasis in colorectal cancer derived organoids, Theranostics, vol. 11, no. 12, p. 5686-5699, 2021. https://doi.org/10.7150/thno.54027
[5] A. Ernst, A. Putscher, T. Samatov, A. Suling, V. Galatenko, M. Shkurnikov, et al. Knockdown of l1cam significantly reduces metastasis in a xenograft model of human melanoma: l1cam is a potential target for anti-melanoma therapy, Plos One, vol. 13, no. 2, p. e0192525, 2018. https://doi.org/10.1371/journal.pone.0192525
[6] C. Flaviana. L1cam expression in human gastrointestinal tract development: from tongue to colon-rectum, Journal of Public Health Research, vol. 12, no. 2, 2023. https://doi.org/10.1177/22799036231165624
[7] W. Christaller, Y. Vos, S. Gebré-Medhin, R. Hofstra, & M. Schäfer. L1 syndrome diagnosis complemented with functional analysis of L1CAM variants located to the two N-terminal Ig-like domains, Clinical Genetics, vol. 91, no. 1, p. 115-120, 2016. https://doi.org/10.1111/cge.12763
[8] M. Marx, S. Diestel, M. Bozon, L. Keglowich, N. Drouot, E. Bouché, et al. Pathomechanistic characterization of two exonic l1cam variants located in trans in an obligate carrier of x-linked hydrocephalus, Neurogenetics, vol. 13, no. 1, p. 49-59, 2012. https://doi.org/10.1007/s10048-011-0307-4