[1] Verschueren, Erik, et al. "The immunoglobulin superfamily receptome defines cancer-relevant networks associated with clinical outcome." Cell 182.2 (2020): 329-344.
[2] Huene, Aidan L., et al. "A family of unusual immunoglobulin superfamily genes in an invertebrate histocompatibility complex." Proceedings of the National Academy of Sciences 119.40 (2022): e2207374119.
[3] Soltantoyeh, Tahereh, et al. "Chimeric antigen receptor (CAR) T cell therapy for metastatic melanoma: challenges and road ahead." Cells 10.6 (2021). 1450.
[4] von Lersner, Ariana, Lenny Droesen, and Andries Zijlstra. "Modulation of cell adhesion and migration through regulation of the immunoglobulin superfamily member ALCAM/CD166." clinical & experimental metastasis 36.2 (2019): 87-95.
[5] Velychko, L. M., et al. "Expression of lymphocyte activation markers CD 54 (ICAM-1), CD 5, CD 95 (FAS) and neutrophil activation marker CD15 in the peripheral blood of patients with intermediate uveitis and healthy individuals." (2021).
[6] Wai Wong, Chee, Danielle E. Dye, and Deirdre R. Coombe. "The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis." International journal of cell biology 2012 (2012).
[7] Kasai, Yutaka, et al. "Trans-homophilic interaction of CADM1 promotes organ infiltration of T-cell lymphoma by adhesion to vascular endothelium." Cancer science 113.5 (2022): 1669-1678.
[8] Funaki, Toko, et al. "CADM1 promotes malignant features of small-cell lung cancer by recruiting 4.1 R to the plasma membrane." Biochemical and Biophysical Research Communications 534 (2021): 172-178.
[9] Salie, Muneeb. Investigating candidate genes identified by genome-wide studies of granulomatous diseases in susceptibility to tuberculosis: ANXA11 and the CADM family. Diss. Stellenbosch: University of Stellenbosch, 2010.
[10] Wikman, Harriet, et al. "Loss of CADM1 expression is associated with poor prognosis and brain metastasis in breast cancer patients." oncotarget 5.10 ( 2014): 3076.
[11] Sakurai-Yageta, Mika, et al. "Tumor suppressor CADM1 is involved in epithelial cell structure." Biochemical and biophysical research communications 390.3 (2009): 977-982.
[12] Watanabe, Toshiki. "Adult T-cell leukemia: molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. "Blood. The Journal of the American Society of Hematology 129.9 (2017): 1071-1081.
[13] Zhang, Wu, et al. "CADM1 regelates the G1/S transition and represses tumorigenicity through the Rb-E2F pathway in hepatocellular carcinoma." Hepatobiliary & Pancreatic Diseases International 15.3 (2016): 289-296.
[14] Si, Xiaoqiang, et al. "CADM1 inhibits ovarian cancer cell proliferation and migration by potentially regulating the PI3K/Akt/mTOR pathway." Biomedicine & Pharmacotherapy 123 (2020): 109717.
[15] Vuletić, Ana, et al. "Cross-talk between tumor cells undergoing epithelial to mesenchymal transition and natural killer cells in tumor microenvironment in colorectal cancer." Frontiers in Cell and Developmental Biology (2021): 3107.
[16] Kuramochi, Masami, et al. "TSLC1 is a tumor-suppressor gene in human non-small-cell lung cancer." Nature genetics 27.4 (2001): 427-430.
[17] Vallath, Sabari, et al. "CADM1 inhibits squamous cell carcinoma progression by reducing STAT3 activity." Scientific Reports 6.1 (2016): 1-12.
[18] Murakami, Shigefumi, et al. "Trans-homophilic interaction of CADM1 activates PI3K by forming a complex with MAGuK-family proteins MPP3 and Dlg." PLoS One 9.2 (2014): e82894.
[19] Lei, Wen, et al. "Tumor suppressor in lung cancer-1 (TSLC1) mediated by dual-regulated oncolytic adenovirus exerts specific antitumor actions in a mouse model." Acta Pharmacologica Sinica 34.4 (2013): 531-540.
[20] Ru Zhang, Zhenglei Xu, and Qinghong Tan. "Expression of CADM1 and DAL-1/4.1 B in Colorectal Cancer. "Chinese Journal of Gerontology 14 (2014): 3845-3847 .
[21] Beaulieu, Jean-François. "Integrin α6β4 in colorectal cancer: expression, regulation, functional alterations and use as a biomarker. "Cancers 12.1 ( 2019): 41.
[22] Cai, Qian, Anding Zhu, and Li Gong. "Exosomes of glioma cells deliver miR-148a to promote proliferation and metastasis of glioblastoma via targeting CADM1." Bulletin du cancer 105.7-8 (2018): 643-651.
[23] Hamashima, Chisato. "Emerging technologies for cervical cancer screening." Japanese Journal of Clinical Oncology 51.9 (2021): 1462-1470.
[24] van Zummeren, Marjolein, et al. "HPV E4 expression and DNA hypermethylation of CADM1, MAL, and miR124-2 genes in cervical cancer and precursor lesions." Modern Pathology 31.12 (2018): 1842-1850.
[25] Wu, Dong-Mei, et al. "Down-regulated lncRNA DLX6-AS1 inhibits tumorigenesis through STAT3 signaling pathway by suppressing CADM1 promoter methylation in liver cancer stem cells." Journal of Experimental & Clinical Cancer Research 38 (2019): 1-17.
[26] Si, Xiaoqiang, et al. "CADM1 inhibits ovarian cancer cell proliferation and migration by potentially regulating the PI3K/Akt/mTOR pathway." Biomedicine & Pharmacotherapy 123 (2020): 109717.
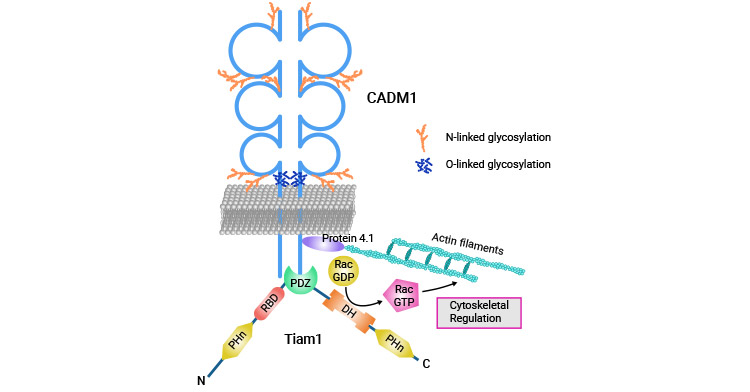
[27] Masuda, Mari, et al. "CADM1 interacts with Tiam1 and promotes invasive phenotype of human T-cell leukemia virus type I-transformed cells and adult T- cell leukemia cells." Journal of Biological Chemistry 285.20 (2010): 15511-15522.
[28] You, Yan, et al. "CADM1/TSLC1 inhibits melanoma cell line A375 invasion through the suppression of matrix metalloproteinases." Molecular Medicine Reports 10.5 (2014): 2621-2626.
[29] Sarkar, Bidhan, et al. "Degradation of p47 by autophagy contributes to CADM1 overexpression in ATLL cells through the activation of NF-κB." Scientific Reports 9.1 (2019): 1-14.
[30] Böhm, Allan, et al. "Molecular mechanisms, diagnostic aspects and therapeutic opportunities of micro ribonucleic acids in atrial fibrillation." International Journal of Molecular Sciences 21.8 (2020): 2742.
[31] Saito-Sasaki, Natsuko, et al. "Cell Adhesion Molecule 1 (CADM1) Is an Independent Prognostic Factor in Patients with Cutaneous Squamous Cell Carcinoma ." Diagnostics 11.5 (2021): 830.

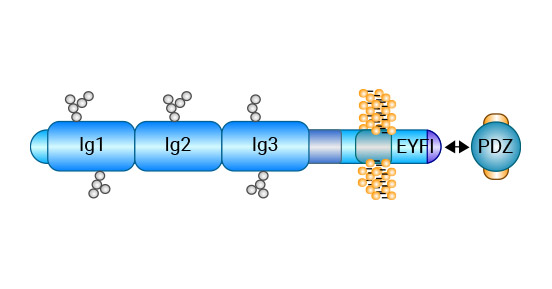
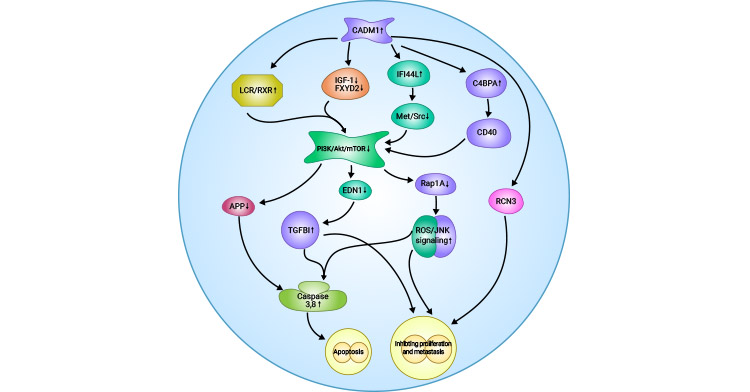
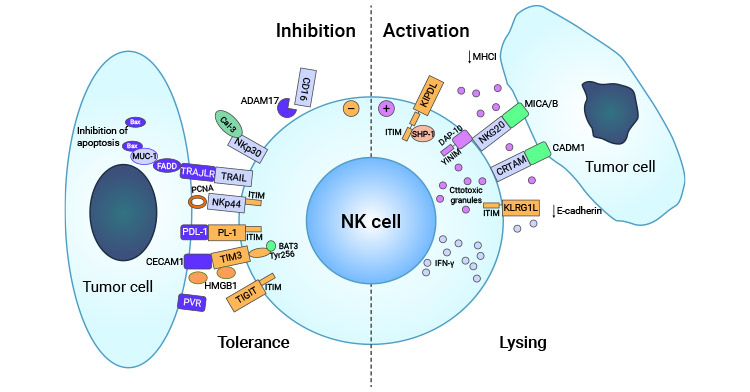
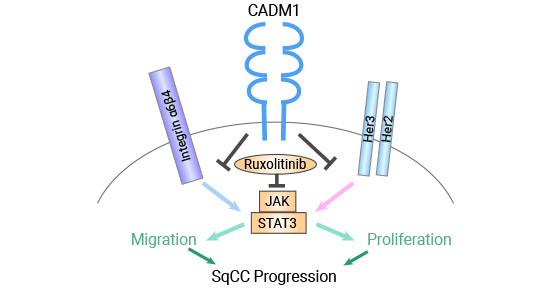



Comments
Leave a Comment