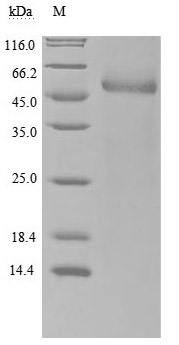
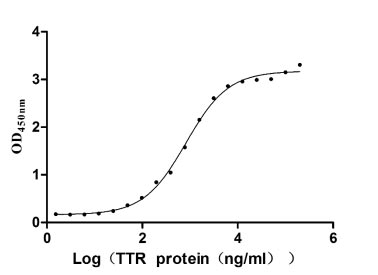
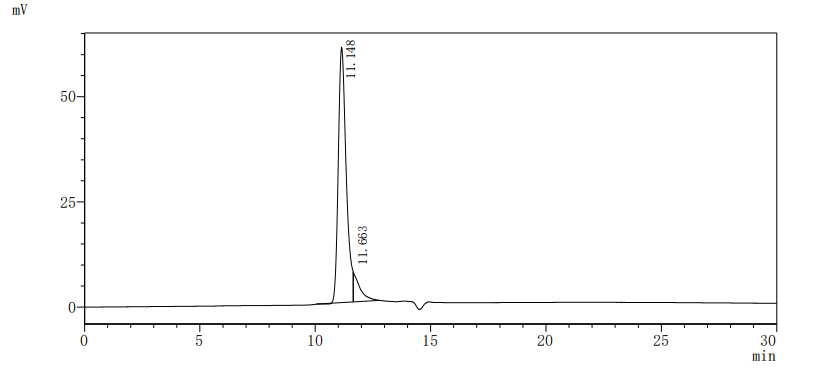
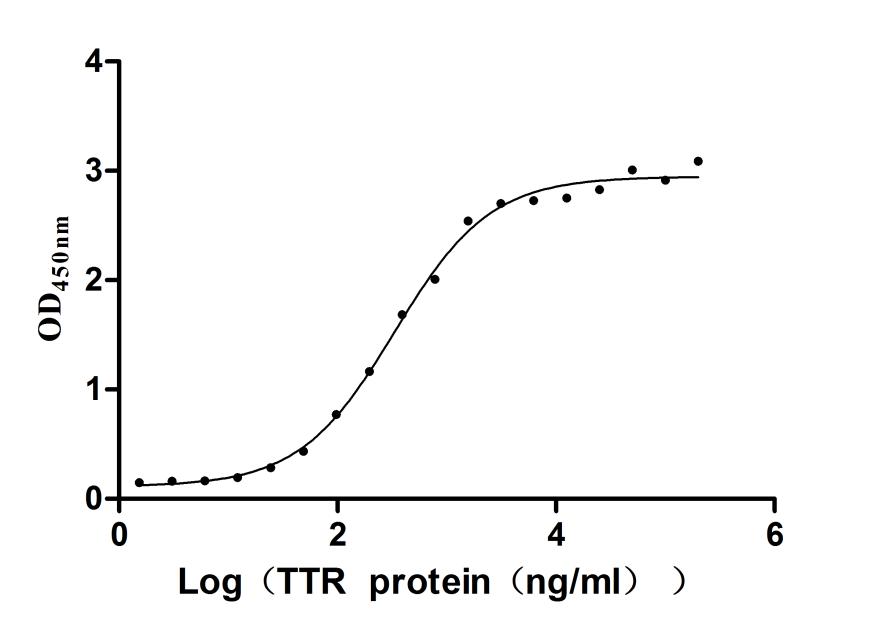
Gene cloning, plasmid construction, protein expression, purification, and analysis are performed to produce the recombinant human RBP4 protein. Primers are designed to amplify the gene sequence encoding the 19-201aa segment of the human RBP4, which is inserted into a plasmid containing the C-terminal hFc-tag gene. After transfecting mammalian cells with the recombinant plasmid, selective antibiotics are used to screen RBP4 protein-expressing cells. The recombinant RBP4 protein is obtained by lysing the cells and purified via affinity chromatography. Its purity exceeds 90% verified by both SDS-PAGE and SEC-HPLC. The LAL test ensures its endotoxin levels are less than 1.0 EU/μg. Functional ELISA demonstrates RBP4’s binding to the TTR (CSB-MP025270HUh6) with an EC50 of 695.0-970.1 ng/mL.
Human RBP4 is primarily synthesized in the liver and adipose tissues and plays a crucial role in the transport of retinol (vitamin A) in the bloodstream. RBP4 binds retinol and facilitates its delivery to various tissues, where it is essential for numerous physiological functions, including vision, immune response, and cellular differentiation [1][2][3]. The transport mechanism involves the formation of a complex with transthyretin (TTR), which stabilizes RBP4 and enhances its delivery efficiency to target tissues [4].
In addition to its role as a transport protein, RBP4 has emerged as a significant adipokine, influencing metabolic processes and insulin sensitivity. Elevated levels of RBP4 have been associated with insulin resistance and type 2 diabetes mellitus [5][6][7]. Studies indicate that RBP4 can impair insulin signaling in skeletal muscle and liver, thereby contributing to the pathophysiology of obesity-related insulin resistance [8][9][10]. Increased RBP4 levels have been linked to reduced expression of glucose transporter type 4 (GLUT4), which is critical for glucose uptake in insulin-sensitive tissues [7][11].
Moreover, RBP4 has been shown to correlate with various components of metabolic syndrome, including obesity, dyslipidemia, and hypertension [12][4]. Patients with type 2 diabetes often have elevated RBP4 levels, which is thought to exacerbate insulin resistance and contribute to the progression of diabetic complications, such as diabetic cardiomyopathy and retinopathy [1][8][13].
References:
[1] H. Shan, Y. Ji, H. Gu, H. Li, J. Zhu, F. Ye, et al. Elevated serum retinol binding protein 4 is associated with the risk of diabetic cardiomyopathy, Reviews in Cardiovascular Medicine, vol. 23, no. 4, 2022. https://doi.org/10.31083/j.rcm2304115
[2] L. Zhang, Y. Cheng, S. Xue, & Z. Xu. The role of circulating rbp4 in the type 2 diabetes patients with kidney diseases: a systematic review and meta-analysis, Disease Markers, vol. 2020, p. 1-12, 2020. https://doi.org/10.1155/2020/8830471
[3] Y. Wang, L. Sun, X. Lin, J. Yuan, W. Koh, & A. Pan. Retinol binding protein 4 and risk of type 2 diabetes in singapore chinese men and women: a nested case-control study, Nutrition & Metabolism, vol. 16, no. 1, 2019. https://doi.org/10.1186/s12986-018-0329-0
[4] T. Olsen and R. Blomhoff. Retinol, retinoic acid, and retinol-binding protein 4 are differentially associated with cardiovascular disease, type 2 diabetes, and obesity: an overview of human studies, Advances in Nutrition, vol. 11, no. 3, p. 644-666, 2020. https://doi.org/10.1093/advances/nmz131
[5] U. Kiernan, D. Phillips, O. Trenchevska, & D. Nedelkov. Quantitative mass spectrometry evaluation of human retinol binding protein 4 and related variants, Plos One, vol. 6, no. 3, p. e17282, 2011. https://doi.org/10.1371/journal.pone.0017282
[6] D. Lee, J. Lee, & J. Im. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents, Metabolism, vol. 56, no. 3, p. 327-331, 2007. https://doi.org/10.1016/j.metabol.2006.10.011
[7] A. Cabré, I. Lázaro, J. Girona, J. Manzanares, F. Marimón, N. Plana, et al. Retinol‐binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes, Journal of Internal Medicine, vol. 262, no. 4, p. 496-503, 2007. https://doi.org/10.1111/j.1365-2796.2007.01849.x
[8] L. Zz, L. Xz, J. Liu, & L. Chen. Serum retinol-binding protein 4 levels in patients with diabetic retinopathy, Journal of International Medical Research, vol. 38, no. 1, p. 95-99, 2010. https://doi.org/10.1177/147323001003800111
[9] N. Shivakumar, M. Kumar, M. Aswathanarayan, M. Venkatesh, M. Sheshadri, S. Deshmukh, et al. Role of retinol-binding protein 4 in obese asian indians with metabolic syndrome, Journal of Medical Biochemistry, vol. 31, no. 1, p. 40-46, 2012. https://doi.org/10.2478/v10011-011-0032-4
[10] M. Schina, J. Koskinas, D. Tiniakos, E. Hadziyannis, S. Savvas, B. Karamanos, et al. Circulating and liver tissue levels of retinol‐binding protein‐4 in non‐alcoholic fatty liver disease, Hepatology Research, vol. 39, no. 10, p. 972-978, 2009. https://doi.org/10.1111/j.1872-034x.2009.00534.x
[11] K. Krzyżanowska, L. Zemany, W. Krugluger, G. Schernthaner, F. Mittermayer, C. Schnack, et al. Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes, Diabetologia, vol. 51, no. 7, p. 1115-1122, 2008. https://doi.org/10.1007/s00125-008-1009-9
[12] M. Eynatten, P. Lepper, D. Liu, K. Lang, M. Baumann, P. Nawroth, et al. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease, Diabetologia, vol. 50, no. 9, p. 1930-1937, 2007. https://doi.org/10.1007/s00125-007-0743-8
[13] N. Alkhouri, R. López, M. Berk, & A. Feldstein. Serum retinol-binding protein 4 levels in patients with nonalcoholic fatty liver disease, Journal of Clinical Gastroenterology, vol. 43, no. 10, p. 985-989, 2009. https://doi.org/10.1097/mcg.0b013e3181a0998d