Transthyretin (TTR) is also known as vitamin A binding protein, pre-albumin (PA), and thyroid-binding pre-albumin. It is a plasma transporter, mainly synthesized by the liver, that binds and transports thyroxine and retinol in the form of a tetramer. TTR can also reflect the synthesis of proteins in the body. It plays an important role in maintaining normal human functions. (See TTR latest progress on Rare Diseases ATTR-CM and ATTR-PN!)
1. TTR Synthesis and Metabolism
Transthyretin (TTR) is a tetramer structure protein, which is mainly synthesized in liver, choroid plexus, pancreas and retinal pigment epithelium. The transthyretin in the blood is secreted by the liver; the TTR in the cerebrospinal fluid is secreted by the choroid plexus; the TTR in the eye is secreted by the retinal pigment epithelium. TTR is mainly degraded in liver (36% ~ 38%), followed by other tissues and organs such as muscle, skin, fat and kidney.
2. The Structure of TTR
TTR gene is a dominant allele, which exists in the 18q11.2-q12.1 region on the long arm of chromosome 18 [1] and contains 4 exons and 3 introns. The 10th amino acid of TTR at the N end of the peptide chain is prone to mutation [2]. TTR is a tetramer composed of four identical subunits bound by noncovalent bonds, each containing 127 amino acids [3]. Unlike the three-dimensional structure of most other proteins, Transthyretin has a very high beta-sheet with only a small alpha helix. Most of the amino acid sequences of TTR are highly conserved, especially the tubular structure at the center of the tetramer, which contains a thyroid hormone binding site.
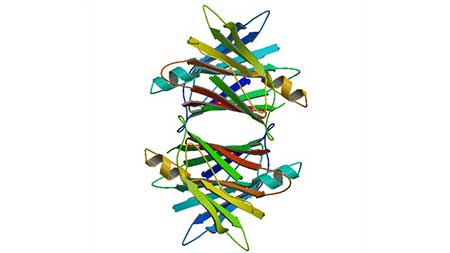
Figure 1. Crystal structure of human transthyretin
3. Physiological Functions of TTR
The main role of TTR in the body is to participate in the transport of thyroxine (mainly T4) and retinol (vitamin A) [4] as a transporter, and participate in maintaining normal levels of retinol, thyroxine and retinol-binding protein in human body [5].
The content of TTR in the cerebrospinal cord and is high. It is the main carrier of T4 and is associated with various central nervous system lesions.
There is also a certain concentration of TTR in human plasma, which is the second binding transporter of thyroid hormone in plasma in addition to thyroid binding globulin.
Thyroxine plays an important role in the development and maintenance of human body. Vitamin A deficiency is also a major cause of night blindness. Therefore, TTR, as a transporter of thyroxine and retinol, can reflect the body's nutrition and some pathological changes, and can be used as an indicator to predict and judge the prognosis of diseases [6].
TTR can accurately reflect the protein synthesis and changes in the body [7]. When proteins in the body are lost and in a state of stress, TTR level decreases rapidly and increases conversely [8].
4. TTR and Diseases Research
TTR has been shown to be associated with a variety of diseases in the human body, such as familial thyroid amyloidosis, diabetes, and the like. TTR misfolding and aggregation are known to be associated with senile systemic amyloidosis [9], familial amyloid polyneuropathy (FAP) [10], familial amyloid cardiomyopathy (FAC) and other amyloidosis diseases.
4.1 Transthyretin Familial Amyloid Polyneuropathy (TTR - FAP)
Under normal physiological conditions (such as pH=7.2), TTR is a highly stable tetramer structure protein. Under pathological or abnormal physiological conditions (such as stress and inflammation), TTR tetramers can be decomposed and degraded into monomers. Its monomer will generate a variety of complex amyloid fibers, which will lead to abnormal physiological aggregation of intracellular amyloid fibers. Amyloid fibers can be deposited in a variety of organs, including nerves, heart and kidney, which will interfere with normal function [11].
Point mutations in the TTR gene are now believed to be the main cause of familial transcriptional thyroxine protein amyloidosis, leading to abnormal deposition of thyroxine amyloidosis fibers and ultimately to severe damage to organs and tissues [12].
Familial thyroid amyloidosis (FAP) is a fatal disease that causes irreversible damage to multiple tissues and organs and ultimately leads to death.
Main symptoms of TTR-FAP patients: sensory loss, lower limb pain, weakness and other neuropathic symptoms; severe autonomic nervous system damage, erectile dysfunction, alternating diarrhea and constipation, unintentional weight loss, orthostatic hypotension, urinary incontinence, delayed gastric emptying and other symptoms [13].
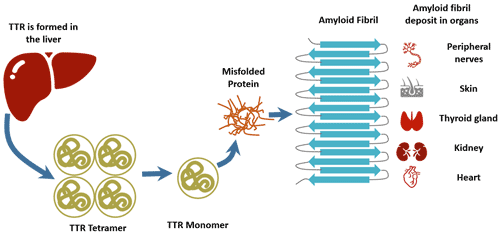
Figure 2. The pathogenesis of TTR-FAP
4.2 Diabetes
Studies have shown that there is a certain correlation between the content of TTR in plasma and the body's blood glucose level. Transthyretin is closely related to type 2 diabetes. It affects type 2 diabetes directly or indirectly through the following aspects: TTR affects type 2 diabetes by damaging B cells, reducing insulin sensitivity, and affecting hormone and adipokine metabolism.
- TTR damage to B cells: B cell damage is associated with the deposition of TTR in B cells. Experiments have shown that the levels of amylin (IAPP) and TTR are increased in plasma of patients with type 2 diabetes, and the function and number of β cells are decreased.
- The relationship between TTR and insulin resistance: TTR is negatively correlated with cellular cell function index (HOMA-IS), indicating that the increase in TTR is positively correlated with insulin resistance.
- Effect of TTR on glucagon: TTR is stored in the secretory vesicles of cells and is secreted together with glucagon. TTR can increase the synthesis and secretion of glucagon and promote the development of type 2 diabetes.
-
The effect of TTR on thyroxine: TTR is the major binding protein of thyroxine. The mutation of TTR can cause the change of serum thyroxine level, which affects blood sugar level and eventually aggravates type 2 diabetes.
On the one hand, TTR forms a TTR-T3-PPAR complex that activates phosphoenolpyruvate carboxykinase, promotes hepatic gluconeogenesis, increases liver glucose output, and aggravates type 2 diabetes.
On the other hand, when TTR is elevated, the clearance rate of T4 is slowed down, the half-life is prolonged, serum free thyroxine is reduced, the effects of sugar and lipid metabolism in muscle and tissues are weakened, glucose in plasma is increased, and type 2 diabetes is aggravated.
- Effect of TTR on Retinol Binding Protein 4 (RBP4): RBP4 is a newly discovered adipokines that are closely related to insulin resistance and the development of type 2 diabetes.
5. Correlation between TTR and Tumor
5.1 Progress of TTR in Pancreatic Cancer Research
Pancreatic cancer (PC) is one of the most common malignancies of the digestive system. Early diagnosis and treatment can significantly improve survival. Abnormal expression of TTR in pancreatic cancer was confirmed [14].
5.2 Progress of TTR in Lung Cancer Research
TTR has been preliminarily confirmed in vitro for the first time that TTR has significant inhibitory effect on the growth of liver cancer cells. TTR gene mutation is related to the occurrence of primary liver cancer.
TTR gene can be used as one of the candidate genes of tumor suppressor genes related to human liver cancer, providing a new option for the treatment of liver cancer at the gene level [15]. At the same time, this inhibitory effect of TTR on liver cancer cells also provides a new idea and train of thought for clinical targeted treatment candidate drugs for liver cancer.
5.3 Progress of TTR in Ovarian Cancer Research
In the diagnosis of ovarian cancer, TTR is considered to be an ideal biomarker. Because transthyretin (TTR) is sensitive to the changes in nutrients and metabolic changes in the body, it is a traditional marker of nutrition, inflammation and stress. TTR combined with other proteins, such as retinol-binding protein and Apo III, can significantly improve the level of auxiliary diagnosis of early ovarian cancer.
5.4 Progress of TTR in Other Malignant Tumors
Colon cancer and rectal cancer are malignant tumors of the digestive tract tumors that are second only to liver cancer and gastric cancer. A diagnostic study of colorectal cancer with a combination of TTR protein and C3a-desArg may provide a new candidate marker for screening colorectal cancer tumor markers [16].
In addition, some experiments have suggested that TTR has a certain diagnostic value for tumors in the brain.
6. Therapeutic Research of Transthyretin-Related Familial Amyloid Polyneuropathy
Regarding the diseases caused by transthyretin, the most studied is the treatment of transthyretin-related familial amyloid polyneuropathy.
TTR-FAP is a rare, progressive and fatal neurodegenerative disease affecting approximately 8,000 patients worldwide [17]. Transthyretin-associated familial amyloidosis multiple neuropathy is caused by the accumulation of TTR monomer, so the radical treatment of ATTR requires blocking or delaying the deposition of amyloid substance.
Reduce the Generation of MT-TTR
The mutant TTR (MT-TTR) is unstable and easily cleaves into monomers. The monomer is mispolymerized to form soluble oligomers to form insoluble amyloid fibers. Amyloid fibers can directly cause compression of tissues and cause disease.
- Liver Transplantation: TTR is mainly produced by liver, and orthotopic liver transplantation (OLT) is the only treatment that can effectively inhibit the synthesis of TTR [18].
- Block TTR Synthesis: DNA sequence needs to be transcribed into mRNA to further synthesize the corresponding protein. Interference with mRNA can inhibit the mutation and endogenous synthesis of wild type TTR. The two technologies currently under study are antisense oligonucleotides (ASO) and small interfering RNA (siRNA).
Stable TTR Tetramer
The dissociation of TTR tetramers is considered to be a rate-limiting step for amyloidosis, so stabilization of TTR tetramers is a treatment for ATTR [19].
There are currently two TTR stabilizers to complete Phase III clinical trials: diflunisal and tafamidis.
- Diflunisal [20]: It is a non-steroidal anti-inflammatory drug that can slow the progression of neurological dysfunction in patients with TTR-FAP and preserve their quality of life.
- Tafamidis: Tafamidis is a new specific TTR stabilizer that is used in the European Union to treat TTR amyloidosis in adult stage 1 symptomatic polyneuropathy to delay peripheral nerve damage. Tafamidis binds to the thyroxine binding site on the TTR tetramer, preventing its dissociation into monomers. In 2011, the European Medicines Regulatory Agency approved chlorpheniric acid for the treatment of patients with early TTR-FAP [21].
Clear Amyloid
The representative drugs for inhibiting or removing amyloid substances are doxycycline and tauroursodeoxycholic acid.
Doxycycline is a tetracycline antibiotic. Animal experiments have shown that it can inhibit the formation of amyloid fibrils, destroy the deposited fibers, and have an effect on both FAP and light chain amyloidosis.
Tauroursodeoxycholic acid (TUDCA) has anti-oxidant and anti-apoptotic effects and can reduce the deposition of pre-TTR fibers. When doxycycline is combined with TUDCA, the deposited amyloid and non-fibrous TTR oligomers are significantly reduced.
Serum amyloid substance P scavenger is also a drug that removes amyloid from tissues. Serum amyloid P component (SAP) is a glycoprotein that recognizes and binds to misfolded polypeptide structures in amyloid deposits, prevents the removal of amyloid deposits, and stabilizes and promotes amyloidosis. In SAP knockout mice, amyloid deposition decreased. Reducing amyloid deposits by removing SAP is an effective treatment. Animal studies have shown that anti-SAP antibodies can rapidly remove large amounts of deposited amyloid in organs by means of complement and macrophage without significant side effects.
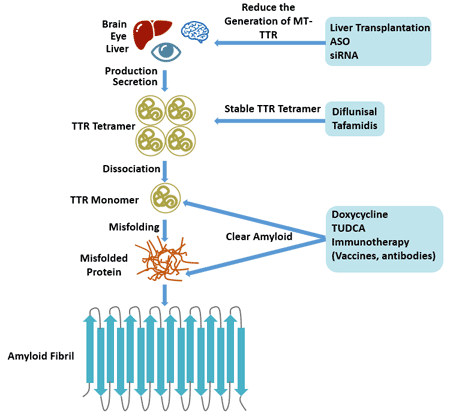
Figure 3. Therapy of transthyretin-related familial amyloid polyneuropathy
7. Clinical Application of TTR
TTR is associated with many pathophysiological processes in the human body.
TTR can be used for early diagnosis of lung cancer and can be used as one of the indicators for lung cancer prognosis and postoperative follow-up. It is expected to improve the clinical diagnosis rate of early stage lung cancer by combining lung cancer-related tumor markers such as CEA and NSE.
In addition, the progression of glomerulonephritis and inflammatory pleural effusion caused by pulmonary infection can be indirectly reflected by monitoring the concentration of TTR in urine and pleural effusion.
In pancreatic cancer, TTR can assist in the diagnosis of early pancreatic cancer together with CA19-9, CA50 and pancreatic protein (PAP), so as to improve the early diagnosis rate. It can also be used to judge the degree of differentiation, atypia and malignancy of pancreatic cancer, and can be used as one of the follow-up indicators of patients after surgery.
The TTR expression level was positively correlated with the postoperative visual acuity [22]. Therefore, TTR can be used as a potential monitoring index for the development of high myopia.
Transthyretin, as a potential serological marker, can also be used in the diagnosis of early rheumatoid arthritis [23].
References
[1] Sparkes R S, Sasaki H, Mohandas T, et al. Assignment of the prealbumin (PALB) gene (familial amyloidotic polyneuropathy) to human chromosome region 18q11.2-q12.1 [J]. Human Genetics, 1987, 75(2): 151-154.
[2] Hamilton J A, M.D. Benson. Transthyretin: a review from a structural perspective [J]. Cellular & Molecular Life Sciences Cmls, 2001, 58(10): 1491-521.
[3] Biroccio A, Boccio P D, Panella M, et al. Differential post-translational modifications of transthyretin in Alzheimer's disease: a study of the cerebral spinal fluid [J]. Proteomics, 2010, 6(7): 2305-2313.
[4] Schweigert F J, Wirth K, Raila J. Characterization of the microheterogeneity of transthyretin in plasma and urine using SELDI-TOF-MS immunoassay [J]. Proteome Science, 2004, 2(1): 5.
[5] Episkopou V, Maeda S, Nishiguchi S, et al. Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone [J]. Proceedings of the National Academy of Sciences, 1993, 90(6): 2375-2379.
[6] Su Y, Jono H, Misumi Y, et al. Novel function of transthyretin in pancreatic alpha cells [J]. Febs Letters, 2012, 586(23): 4215-4222.
[7] Almeida M, Gales L, Damas A, et al. Small Transthyretin (TTR) Ligands as Possible Therapeutic Agents in TTR Amyloidoses [J]. Current Drug Target - CNS & Neurological Disorders, 2005, 4(5): 587-596.
[8] Gouvea I E, Kondo M Y, Assis D M, et al. Studies on the peptidase activity of transthyretin (TTR) [J]. Biochimie, 2013, 95(2): 215-223.
[9] Westermark P, Sletten K, Johansson B, et al. Fibril in senile systemic amyloidosis is derived from normal transthyretin [J]. Proceedings of the National Academy of Sciences, 1990, 87(7): 2843-2845.
[10] Coelho T. Familial amyloid polyneuropathy: new developments in genetics and treatment [J]. Current Opinion in Neurology, 1996, 9(5): 355-9.
[11] Hou X, Aguilar M I, Small D H. Transthyretin and familial amyloidotic polyneuropathy: Recent progress in understanding the molecular mechanism of neurodegeneration [J]. FEBS Journal, 2007, 274(7): 1637-1650.
[12] Sekijima Y. Transthyretin (ATTR) amyloidosis: clinical spectrum, molecular pathogenesis and disease-modifying treatments [J]. Journal of Neurology, Neurosurgery & Psychiatry, 2015: jnnp-2014-308724.
[13] Elisabeth Jonsèn, Athlin E, Suhr O. Familial amyloidotic patients’ experience of the disease and of liver transplantation [J]. Journal of Advanced Nursing, 1998, 27(1): 7.
[14] Lv S, Gao J, Zhu F, et al. Transthyretin, identified by proteomics, is overabundant in pancreatic juice from pancreatic carcinoma and originates from pancreatic islets [J]. Diagnostic Cytopathology, 2011, 39(12): 875-881.
[15] Schweigert F J, Sehouli J. Transthyretin, a biomarker for nutritional status and ovarian cancer [J]. Cancer Research, 2005, 65(3): 1114.
[16] Clarke C H, Yip C, Badgwell D, et al. Proteomic biomarkers apolipoprotein A1, truncated transthyretin and connective tissue activating protein III enhance the sensitivity of CA125 for detecting early stage epithelial ovarian cancer [J]. Gynecologic Oncology, 2011, 122(3): 548-553.
[17] Ando Y, Nakamura M, Araki S. Transthyretin-Related Familial Amyloidotic Polyneuropathy [J]. Archives of Neurology, 2005, 62(7): 1057.
[18] Ruberg F L, Maurer M S, Judge D P, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: The Transthyretin Amyloidosis Cardiac Study (TRACS) [J]. American heart journal, 2012, 164(2): 222-228.e1.
[19] Hammarstrom P. Prevention of Transthyretin Amyloid Disease by Changing Protein Misfolding Energetics [J]. Science, 2003, 299(5607): 713-716.
[20] Tojo K, Sekijima Y, Kelly J W, et al. Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis [J]. Neuroscience Research, 2006, 56(4): 441-449.
[21] Ando Y, Coelho T, Berk J L, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians [J]. Orphanet Journal of Rare Diseases, 2013, 8(1): 31.
[22] Van A E, De Letter E A, Veckeneer M, et al. Transthyretin levels in the vitreous correlate with change in visual acuity after vitrectomy [J]. Acta Neuropsychiatrica, 2010, 21(s2): 0-0.
[23] Ni M, Wei W, Feng Q, et al. Transthyretin as a potential serological marker for the diagnosis of patients with early rheumatoid arthritis [J]. Clinical & Experimental Rheumatology, 2013, 31(3): 394-399.
CUSABIO team. Knowledge about Transthyretin. https://www.cusabio.com/c-20868.html







Comments
Leave a Comment