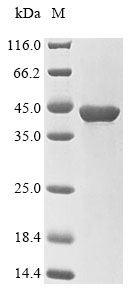
This Human PPP1CC recombinant protein was produced in E.coli, where the gene sequence encoding Human PPP1CC (2-323aa) was expressed with the N-10xHis tag and C-Myc tag. The purity of this PPP1CC protein was greater than 85% by SDS-PAGE.
PPP1CC is a member of the protein phosphatase 1 (PP1) family, which is a serine/threonine protein phosphatase. Its primary function is to remove phosphate groups from proteins, thereby regulating the activity of various cellular signaling pathways. PPP1CC's substrates within the cell include protein kinases, phosphorylated proteins, and more. By dephosphorylating these substrates, it can influence essential biological processes such as cell growth, differentiation, apoptosis, and metabolism.
PPP1CC interacts with multiple proteins, including cyclin-dependent kinases, apoptosis-regulating proteins, nuclear factors, and more. These interactions allow it to modulate the activities of these proteins, thereby controlling cellular behaviors.
Abnormal expression or mutations in PPP1CC are associated with the development of various diseases. For example, it is linked to the development of certain tumors because it can affect the regulation of the cell cycle. Additionally, abnormal activity of PPP1CC is also associated with some neurological disorders as it can impact the function of neurons.