What Is Cellular Senescence?
In 1961, Leonard Hayflick reported that human fibroblasts cultured in vitro multiplied only a limited number of times, rather than reproducing limitlessly as previously thought. The limited-time of the proliferation of cells is the "Hayflick Boundary." This is also the first theory of aging.
Cellular senescence, also known as cell aging, is a highly stable cell cycle arrest or withdrawal that occurs under different stressful conditions. And cellular senescence is ubiquitous in various organisms. It can permanently inhibit growth and prevent cell proliferation. The cells in the quiescent phase just temporarily exit the cell cycle and they will enter the cell cycle once they receive mitogenic signal. However, the growth arrest of senescent cells is irreversible. It means that senescent cells cannot re-enter the cell cycle even under favorable growth conditions. But their metabolism is still ongoing.
Cellular Senescence and Individual Aging
Cellular senescence refers to the senescence of one or more cells. Individual aging is the aging of living organisms. For single-celled organisms, cellular senescence is almost synchronous with individual aging. However, the cellular aging of multicellular organisms does not mean that individuals will age. Individual aging can reflect that most cells are senescent to some extent.
Characteristics of Senescent Cells
A distinct feature of aging cells is their stable cell cycle arrest. Senescent cells normally undergo chromatin rearrangement, metabolic reprogramming such as the increase of glycolysis, mitochondrial function, and autophagy. Aging cells become flattened, enlarged, and vacuolated. Senescent cells also form cytoplasmic bridges that allow them to signal neighboring cells through intercellular proteins.
Advantages and Disadvantages of Cellular Senescence
The cellular aging pathway is activated by various triggers. In the short term, cellular senescence exhibits beneficial aspects such as the promotion of embryonic development & wound healing and tumor suppression. However, in the late stage of cell injury, cellular aging brings catastrophic consequences like promoting tumorigenesis and inducing aging-related diseases.
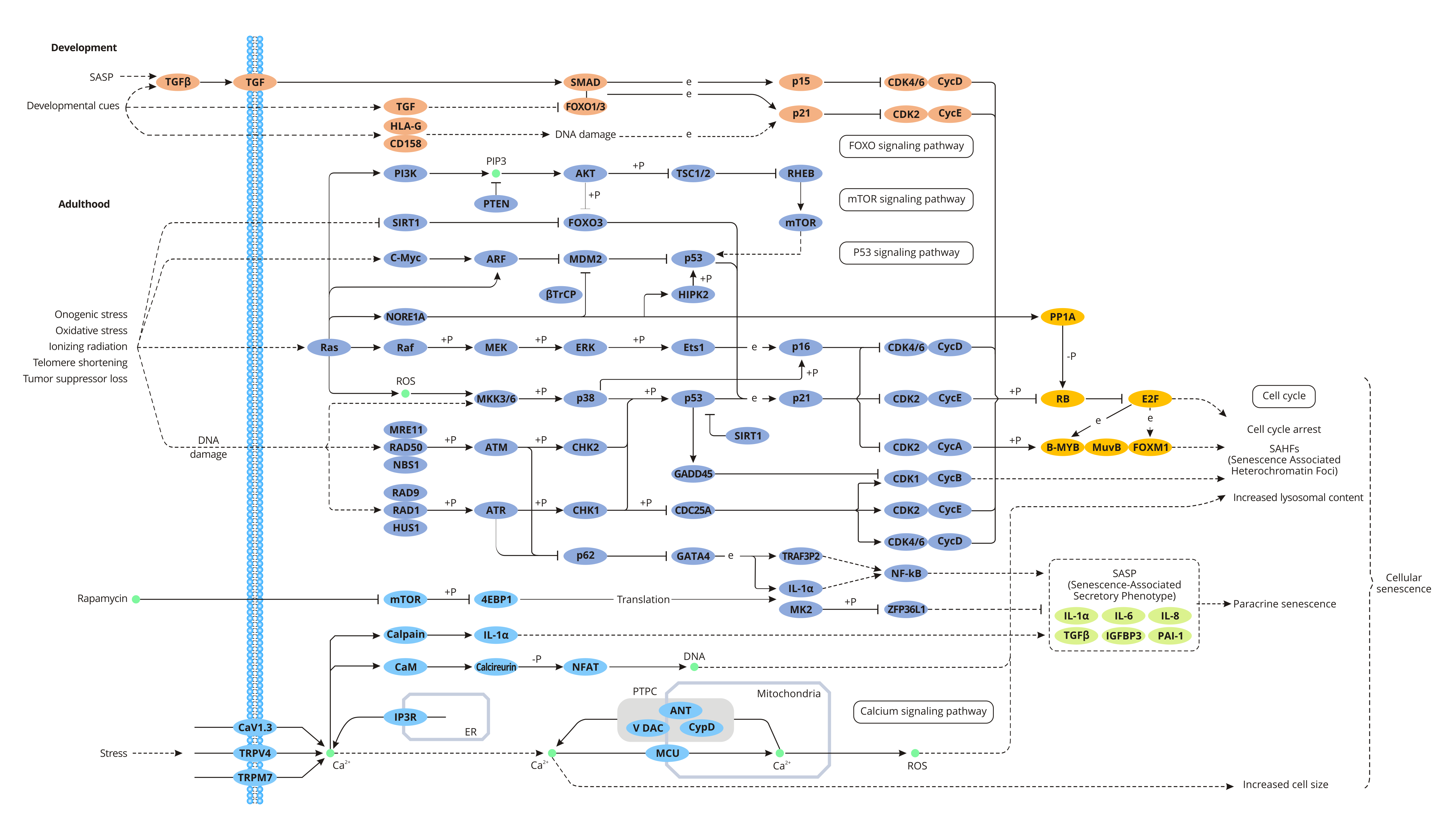
The Signaling Pathways of Cellular Senescence
During cell senescence, two tumor suppressor pathways - p53/p21CIP1 and p16INK4a/Rb are responsible for cell growth arrest.
P53/p21CIP1 pathway
Inducing factors of cellular senescence such as telomere loss & the DNA damages caused by carcinogenic or oxidative stress. DNA damages activate a kinase cascade of ATM/ATR/CHK1/CHK2, which ultimately leads to activation of p53. P53 induces transcription of the cyclin-dependent kinase inhibitor (CDKI) p21CIP1. p21CIP1 and then inhibits CDK4/6 activity, resulting in the conversion of the phosphorylated Rb protein to a low phosphorylated or dephosphorylated form. The non-phosphorylated Rb protein binds to the transcriptional regulator E2F, preventing E2F from activating the genes expression that is necessary for the cell cycle. Therefore, cells cannot enter the S phase through the G1 checkpoint, resulting in C0/G1 cell arrest, which initiates cellular senescence.
p16INK4a/Rb Pathway
p16INK4a inhibits CDK4/6-cyclin D activity by binding to CDK4/6, causing a conformational change in CDK4/6. Inactivated CDK4/6-cyclin D inhibits phosphorylation of Rb, enhancing the binding of Rb to E2F. The process inhibits the cell cycle from G1 to S phase. Studies have found that the senescent cells mainly contain the G1 phase DNA content. So it is considered that the senescent cells are arrested in the G1 phase. The senescent cells cannot successfully enter the S phase to initiate chromosome replication to complete the cell cycle.
Cellular Senescence and Diseases
Since cell aging is associated with a large number of diseases and pathological processes, so many studies are focused on controlling diseases by promoting or inhibiting cell senescence. Some methods have been successful in mouse disease models, and some excellent clinical studies are ongoing. Bcl-2 (B-cell lymphoma 2) is a member of the Bcl-2 family of regulator proteins that modulate apoptosis by inhibiting anti-apoptotic proteins or inducing pro-apoptotic proteins. Inhibitors of BCL-2 are widely used to induce apoptosis in senescent cells. Besides, drugs that induce cellular senescence can also be used as anticancer drugs, including CDK4/6 inhibitors such as palbociclib.
Many studies have confirmed that cell aging plays an important role in the development of chronic diseases, organ failure, and poor prognosis. Therefore, the markers of cell aging are expected to be used for disease diagnosis. And cell senescence may also be a potential drug target for disease treatment. Cellular aging also has a beneficial side for chronic diseases. For example, senescent hepatic stellate cells and cardiac fibroblasts can reduce fibrosis of the corresponding organs and limit disease progression.





