According to the statistics from the World Health Organization (WHO), approximately 300 million people worldwide suffer from hepatitis B. Hepatitis B-caused liver complications including cirrhosis, liver failure, and hepatocellular carcinoma are the direct causes of 80% of primary liver cancers worldwide and contributes to nearly one million deaths each year. In our country, there are about 100,000 carriers of the hepatitis B virus, and the incidence of hepatitis B continues to rise, with about 300,000 people dying every year from HBV-related cirrhosis.
Hepatitis B is a serious form of hepatitis caused by the hepatitis B virus, which has been included in the list of class I carcinogens by the International Agency for Research on Cancer (IARC) of the WHO. Some hepatitis B patients only experience short-term illness referred to as acute infection, while other patients develop chronic hepatitis B, a severe, lifelong condition.
1. What Is Hepatitis B Virus?
In 1963, Baruch Blumberg accidentally discovered that a protein in the blood serum of an Australian Aboriginal person could react with the serum of a hemophiliac in New York. Hemophiliacs develop a wide array of antibodies due to frequent blood transfusions. Baruch Blumberg named the unknown antigenic substance in the blood of Australian Aborigines the "Australian antigen".
In 1966, Baruch Blumberg et al. detected Aa in the serum of a 12-year-old boy with Down syndrome and hepatitis. This first established the association of the Aa with hepatitis.
By 1967, Baruch Blumberg et al. recognized that Australian antigen was involved in the formation of hepatitis B, making it clear that this antigen was associated with hepatitis B. They also thought that hepatitis may be transmitted through blood transfusion.
In 1970, the Australian antigen was identified as a structural component of HBV. That year scientist D. S Dane and his colleagues observed complete HBV particles in serum, also known as Dane particles [1].
In 1971, scientists isolated the HBV and found that HBV was composed of an outer envelope and an inner core.
In 1972, scientists realized that e antigen (HBeAg) is part of the core of HBV and related to viral infectivity, and identified HBV as a DNA virus.
In 1981, a more sophisticated plasma-derived hepatitis BV vaccine was approved for human use. This vaccination was no longer recommended in 1990, and it is no longer offered in the United States.
The second generation of DNA recombinant hepatitis B vaccines was developed in 1986. These new vaccines are synthetically made and do not contain blood materials, making it impossible to contract hepatitis B from them.
In 1986 HBV was included in the family hepatophilic DNA viruses.
In order to commemorate Baruch Blumberg, the discoverer of the hepatitis B virus, the WHO decided in May 2010 to change the annual World Hepatitis Day from May 19 to July 28 from 2011.
HBV is a partially double-stranded DNA virus and is classified in the family Hepadnaviridae, genus Orthohepadnavirus, that is species-specific and hepatophilic. HBV is only susceptible to humans, chimpanzees, and treeshrews. A complete hepatitis B virion, also known as a Dane particle, is only 42 nm in diameter. HBV can be detected within 30 to 60 days of infection. It has a significant likelihood of developing into a chronic disease in young children.
2. Hepatitis B virus Structure and Components
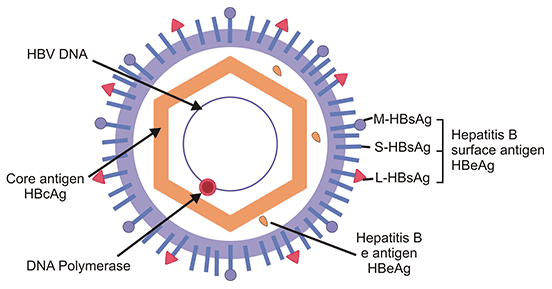
A mature HBV particle is a 42 nm-diameter, enveloped, icosahedral virus. The viral outer envelope is made up of hepatitis B surface antigen (HBsAg) and host-derived lipids without viral nucleic acids and are therefore noninfectious [2]. HBsAg is the basic component of the hepatitis B vaccine. And the spherical envelope surrounds an inner nucleocapsid containing hepatitis B core antigen (HBcAg) complexed with virally encoded polymerase and the viral DNA genome. The core of the HBV also includes e antigen (HBeAg).
Figure 1. HBV structure and components
This picture is from Wikipedia
The genome of HBV is a partially double-stranded circular DNA with about 3.2 kb pairs. The viral genome encodes four overlapping open reading frames (ORFs): S, C, P, and X.
The S ORF encodes the HBsAg and can be structurally and functionally divided into the pre-S1, pre-S2, and S regions. The S domain also contains a glucocorticoid-responsive element (GRE) sequence [3].
The C ORF possesses the pre-core and core regions, which initiate different translations and thus give rise to hepatitis B e antigen (HBeAg) and nucleocapsid HBcAg, respectively. The core gene contains a polyadenylation signal. HBcAg is the organizing framework for the virion and is essential for viral replication and genome packaging. HBeAg is not incorporated into virions and is secreted from the cells. And HBeAg is related to viral replication and high infectivity.
The P ORF overlaps the entire S gene and encodes the viral DNA polymerase (POL), an 800 aa-large protein, which is functionally divided into three domains: the terminal protein domain, which takes part in encapsidation and initiation of minus-strand synthesis, the reverse transcriptase (RT) domain, which catalyzes genome synthesis, and the ribonuclease H domain, which degrades pregenomic RNA and promotes replication.
The X ORF encodes the HBx protein (HBxAg), which is a non-structural regulatory protein with multiple functions, including signal transduction, transcriptional activation, DNA repair, and inhibition of protein degradation [4-7]. HBxAg plays an essential role in productive HBV infection in vivo and may contribute to the oncogenic potential of HBV.
Other functionally important elements within the HBV genome include two direct repeats DR1 and DR2 in the 5' ends of the plus strand, which are required for strand-specific DNA synthesis during the HBV replication [8]. Two enhancer elements En1 and En2 confer a live-specific expression of the complete viral transcripts [9]. A posttranscriptional regulatory element lies in En1 and part of HBxAg ORF [10].
3. How Does Hepatitis B Virus Work?
The pathogenic mechanism of HBV has not been studied clearly now. Some research shows that HBV does not kill the liver cells directly but rather than leading to an abnormal immune response, thus causing secondary inflammation and liver cell damage. But it is still unknown how the virus achieves immune escape. Here mainly introduce the life cycle of HBV.
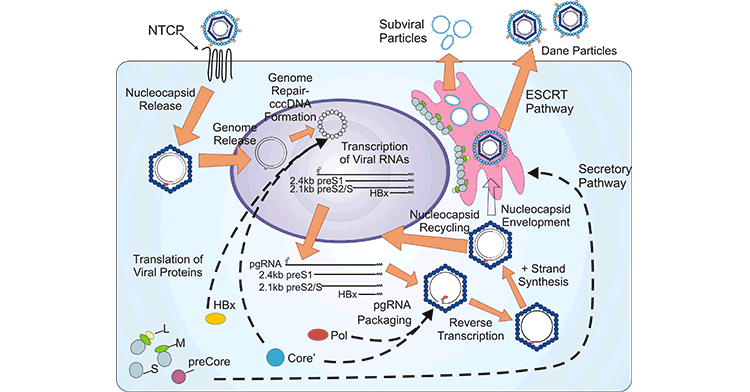
The viral proteins promote the completion of the HBV life cycle, which involves virus entry, covalently closed circular DNA (cccDNA) synthesis, progeny nucleocapsid generation, virion formation, and virion egress, in conjunction with host factors.
A mature HBV virion attaches to the host hepatocyte cell membrane through the sodium taurocholate cotransporting polypeptide receptor (NTCP) on the cell membrane. Viral entry is also mediated by heparin sulfate proteoglycans (HSPGs, such as glypican 5) and EGFR on the surface of hepatocytes.
Upon release from the viral envelope, the nucleocapsid is transported to the cell cytoplasm, where the partially double-stranded DNA genome is converted into cccDNA by the HBV POL. cccDNA biosynthesis is an essential process to establish infection. cccDNA can serve as the template for pre-genomic, pre-core mRNAs, and several sub-genomic mRNAs, which are translated to multiple viral proteins, including HBsAg, HBcAg, HBx, HBV POL, and core-antigen associated proteins.
The binding of HBV POL to pre-genomic mRNA initiates the progeny nucleocapsid generation, which causes the encapsidation and synthesis of relaxed circular DNA rcDNA. The produced nucleocapsids can be re-imported into the nucleus, and the rcDNAis repaired to form cccDNA and maintain cccDNA pool through the intracellular amplification pathway. Alternatively, the resultant nucleocapsids are enveloped and secreted through the endosomal sorting complex required for the transport (ESCRT) pathway.
Figure 2. Schematic representation of the HBV life cycle
This picture is cited from: https://hrjournal.net/article/view/1318
4. How Is Hepatitis B Virus Transmitted?
There are three main modes of transmission of HBV, including blood transmission, mother-to-child transmission, and sexual transmission. HBV is infectious outside the body for seven or more days.
Blood transmission
Infection occurs through blood transfusion, blood products, exposure to instruments contaminated with hepatitis B, and unsafe injections (especially needle sharing among drug users).
In addition, shaving, pedicure, ear piercing, tattooing, filling teeth, and using shared dental appliances and razors are also easily overlooked infection routes in daily life.
Mother-to-child transmission
Mothers infected with hepatitis B can transmit the virus to their newborns through blood or body fluids during the perinatal period (28 weeks gestation to 1 week postpartum).
Sexual transmission
A person who had unprotected sexual intercourse with a hepatitis B-positive partner can become infected with HBV.
Many people stay away from hepatitis B patients because they are afraid of being infected. In fact, hepatitis B is not transmitted through the respiratory tract and digestive tract, nor through the bites by mosquitoes and bed bugs. Contacts without blood exposure, such as working in the same office, shaking hands, hugging, eating in the same restaurant, and using the same public restroom, do not cause transmission.
Family members or close contacts of hepatitis B patients should check the five hepatitis B items as soon as possible. If there are no protective antibodies, they should be injected with the hepatitis B vaccine as soon as possible. At present, the injection of the hepatitis B vaccine is the most effective way to prevent HBV infection. During Acute infection, about 90-95% of patients can be cured, while the vast majority of chronic infections just carry viruses and do not have serious pathological changes. Only 5-10% of patients from chronic infections may have chronic hepatitis.
5. How to Read Hepatitis B Test Results?
The five tests for hepatitis B are serological markers for detecting HBV in the blood. The five items of hepatitis B, also known as two and a half of hepatitis B, include: HBsAg, anti-HBs (HBsAb), HBeAg, anti-HBe (HBeAb), and anti-HBc (HBcAb). Different combinations of the five hepatitis B items are commonly used clinically to judge the status and outcome of infection.
Table 1: Meanings of various combinations of Hepatitis B five test in clinical examination
| HBsAg |
Anti-HBs |
HBeAg |
Anti-HBe |
Anti-HBc |
Clinical Meaning |
| - |
- |
- |
- |
- |
Not infected with HBV in the past and at present. |
| - |
- |
- |
- |
+ |
Infected with HBV before, acute infection at recovery stage. |
| - |
- |
- |
+ |
+ |
Infected with HBV in the past and at present. |
| - |
+ |
- |
- |
- |
Preventive vaccination or HBV infection has been recovered. |
| - |
+ |
- |
+ |
+ |
Previous infection; Acute HBV infection at recovery stage. |
| - |
+ |
- |
- |
+ |
Previous infection; Acute HBV infection gas been recovered. |
| + |
- |
- |
- |
+ |
Acute HBV infection; Chronic HBsAg carriers. |
| + |
- |
- |
+ |
+ |
Acute HBV infection is going to be recovered; Chronic HBsAg carriers, Weak infection; prone to canceration for a long term. |
| + |
- |
+ |
- |
+ |
Acute hepatitis B or chronic hepatitis B, highly contagious. |
| + |
- |
- |
- |
- |
Acute HBV infection in early stage, HBsAg carriers. |
| + |
- |
+ |
- |
- |
Acute HBV infection in early stage, highly contagious. |
| - |
- |
+ |
+ |
+ |
Acute infection in mid-term. |
| + |
- |
+ |
+ |
+ |
Acute infection is going to be recovered; Chronic carriers. |
| + |
- |
- |
+ |
- |
Acute infection is going to be recovered. |
| - |
- |
- |
+ |
- |
Acute infection is going to be recovered. |
| - |
+ |
- |
+ |
- |
HBV infection has been recovered. |
(Note: “+” indicates presence, “-” indicates absence)
The research of liver disease keeps new breakthrough. Basic research plays an important role in it. CUSABIO is committed to scientific progress, providing a series of hepatitis virus kit.
References
[1] Dane DS, Cameron CH, Briggs M. Virus-like particles in serum of patients with australia-antigen-associated hepatitis [J]. Lancet. 1970;1(7649):695–98.
[2] Gavilanes F, Gonzales-Ros A, Peterson D. Structure of hepatitis B surface antigen: characterization of the lipid components and their association with the viral proteins [J]. J Biol Chem. 1982;257:7770–7777.
[3] Tur-Kaspa R, Burk R, et al. Hepatitis B virus DNA contains a glucocortcoid response element [J]. Proc Natl Acad Sci U S A. 1986;83:1627–1631.
[4] Zhang Z, Torii N, et al. X-deficient woodchuck hepatitis virus mutants behave like attenuated viruses and induce protective immunity in vivo [J]. J Clin Invest. 2001;108:1523–1531.
[5] Cross JC, Wen P, Rutter W. Transactivation by hepatitis B virus X protein is promiscuous and dependent on mitogen-activated cellular serine/threonine kinases [J]. Proc Natl Acad Sci U S A. 1993;90:8078–8082.
[6] Hu Z, Zhang Z, et al. Altered proteolysis and global gene expression in hepatitis B virus X transgenic mouse liver [J]. J Virol. 2006;80:1405–1413.
[7] Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus [J]. J Virol. 2004;78:12725–12734.
[8] Seeger C, Ganem D, Varmus HE. Genetic and biochemical evidence for the hepatitis B virus replication strategy [J]. Science. 1986;232:477–485.
[9] Yee J. A liver-specific enhancer in the core promoter region of human hepatitis B virus [J]. Science. 1989;246:658–670.
[10] Huang J, Liang TJ. A novel hepatitis B virus (HBV) genetic element with Rev response element-like properties that is essential for expression of HBV gene products [J]. Mol Cell Biol.
1993;13:7476–7486.
CUSABIO team. What Is Hepatitis B Virus?. https://www.cusabio.com/c-20128.html




Comments
Leave a Comment