Accurate protein quantification is a critical step in most experiments and workflows where proteins are extracted, isolated, and analyzed by biochemical methods. Analyzing the interactions between
biological molecules are important to understand their functions. Describing the binding of proteins to other proteins, nucleic acids, or small molecules is fundamental to biochemical research and has
applications in many other fields, including drug discovery and development.
The purpose of this review is mainly to introduce how to properly determine protein concentration. We emphasize the importance of protein concentration determination, while also, at the same time,
describing in detail different determination approaches and highlighting points of concern. We want to underline the clear difference of these different measurement methods, in terms of their advantages
and disadvantages, respectively.
1. What Is the Protein Concentration?
The concentration of protein is defined as the total amount or content of a specific protein or an array of different proteins in a sample.
2. Why Protein Concentration Is Quantified ?
Protein concentration determination is necessary and widely used in protein biology and molecular biology. Protein quantitative analysis involves many fields and industries
of production and scientific research and is the most popular method in biology, food inspection and adulteration, clinical inspection, disease diagnosis, and quality inspection. The concentration of the
protein sample must be calculated prior to separation, purification, and analysis.
Protein quantification is indispensable to have a better understanding of the total protein content in a sample or in a formulated product. It is an important procedure in any experiment workflow
involving protein extraction or analysis. Precise protein concentrations are key to producing reliable and optimal data for subsequent experiments.
3. Which Methods Are Available to Measure Protein Concentration?
There are many kinds of quantitative methods for protein, among which there are six common ones, including Kjeldahl method, Biuret method, Lowry assay, BCA assay,
Bradford assay, and Ultraviolet (UV) Absorbance at 280 nm.
The measurement of protein concentration is mainly based on two principles: one is to use the common characteristics of the protein, such as nitrogen, peptide bond, refractive index, etc., and the
other is to use specific amino acid residues, basic or acidic groups and aromatic groups in protein to determine protein content.
3.1 Kjeldahl Method
The Kjeldahl method was developed by Johann Kjeldahl in 1883. It includes three steps digestion, distillation, and titration. In this method, the total organic nitrogen is
coverted to ammonium sulfate after digestion of protein with concentrated acid and catalyst. NaOH is added to neutralize the digestate and convert the ammonium to ammonia. Ammonia is distilled off and
collected in the receiving bottle of excess boric acid to form ammonium borate. The total nitrogen content of the sample is measured by titrating the residual boric acid with a suitable titration technique. A
conversion factor (F) is required to convert the measured nitrogen concentration to a protein amount.
3.2 Colorometric Assays
Colorimetric assays methods are based on the generation of a colored solution in the visible spectrum in response to protein. The color change is in proportion to the protein
concentration and is measured by absorbance photometry at a certain wavelength. Typically, a standard curve drawn by standard solutions of bovine serum albumin (BSA) is used to determine protein
amount. Colorimetric assays include the biuret test, Lowry assay, bicinchoninic acid (BCA) assay, and Coomassie blue G-250 dye-binding (Bradford) assay.
3.2.1 Biuret Method
The biuret test, also called Piotrowski's test, is a chemical method for determining the total protein in a sample. Because the structure of peptide bond (-CONH-) in protein molecule is similar to that
of biuret, so proteins can also react with cupric ion (Cu2+) in alkaline solution to generate purplish-red compound.
In the biuret method, a Cu2+ is reduced by two peptide bonds within proteins and then chelated into a violet-colored complex. The color intensity of the product is proportional to the
protein concentration and is measured by absorption spectroscopy at 540 nm.
Later, the biuret test was improved and then developed into two colorimetric analyses of peptides: the Lowry assay and the BCA assay.
3.2.2 Lowry Assay
Lowry assay was first proposed by Oliver Lowry in 1951 [1]. In the Lowry method, peptide bonds first reduce the copper ions into cuprous ions under alkaline conditions. Folin-Ciocalteu
reagent subsequently reacts with cuprous ions and the side chains of tyrosine, tryptophan, and cysteine, producing a dark blue complex known as heteropolymolybdenum Blue. The color intensity of the
heteropolymolybdenum Blue is positively correlated to the protein content in the sample and is measured by absorbance at 660 nm.
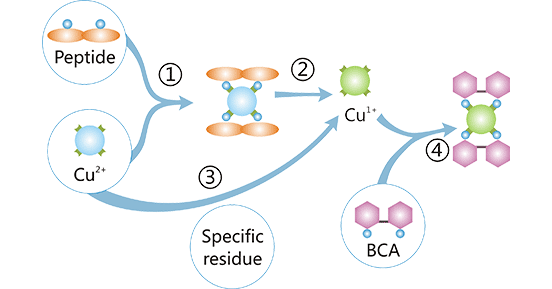
3.2.3 BCA Assay
The BCA assay is the improvement based on the Lowry assay. In the BCA assay, the protein peptide bonds chelate Cu2+ ions and reduce them to cuprous ions (Cu+) ions under alkaline
conditions [2]. The Cu+ ions react with BCA to form a purple complex, the color intensity of which is measured by absorbance photometry at 562 nm [2] [3]. The
absorbance has a good linear relationship with the protein concentration over a wide range.
Figure 1: Principle of the BCA assay
3.2.4 Bradford Assay
The Bradford assay, first developed by Marion M. Bradford in 1976 [4], is a dye-binding method that is based on the color change induced by Coomassie's brilliant blue G-250 dye-protein
interaction. Coomassie G-250 dye reacts with ionizable groups on the protein, leading to changes in protein conformation and subsequent exposure of hydrophobic pockets [4] [5]. The dye
binds to the hydrophobic amino acids, forming stable and blue complexes. The color intensity of the blue complexes can be measured by absorbance photometry at 595 nm. The relationship of absorbance
to protein concentration is linear.
3.3 Ultraviolet (UV) Absorbance at 280 nm
It is a direct method for determining protein concentration by measuring the UV absorbance of a sample at 280 nm (A280). Tyrosine, tryptophan, and other aromatic amino
acids in proteins can absorb ultraviolet light [6]. The absorption peak of these aromatic amino acids is at 280mm wavelength, and the optical density of the absorption peak is proportional to
their concentration. So it can be used as the basis for the quantitative determination of protein. Due to the different contents of tyrosine and tryptophan in various proteins, it is necessary to know its
extinction (ε) coefficient as a reference for accurate quantification. The ε coefficient can be experimentally determined or calculated from the amino acid sequence.
3.4 Enzyme-linked immunosorbent assay (ELISA)
In the ELISA, the target protein is captured by a specific antibody labeled with an enzyme and then quantified using an enzyme reaction with its substrate. The light
absorption of the produced complex is measured via the microplate reader at a certain wavelength. The intensity of the color produced by the enzyme-substrate reaction is proportional to the protein
concentration in the sample. The protein concentration is calculated by referring to the standard curve. Now there are various premium quantitative ELISA kits in the market, which provide more convenience
for the quantification of specific proteins.
3.5 Protein Assay Kits
Based on the principles of protein quantification mentioned above, various protein assay kits are also gradually available, mainly including BCA protein assay Kits and Lowry
protein assay Kits, which realize the simple, high stability, high sensitivity and high compatibility of protein concentration determination.
4. The Differences among Methods of Protein Concentration Determination
PWhen determining the protein content in a sample, the first consideration is to select an appropriate measurement method. Since there are a broad variety of protein assays
available, we need to take a number of factors into account to ensure that the assay used is most suitable for the application. Each assay has its own advantages and limitations. It may also be necessary to
obtain more than one type of protein assay for research applications since there is not one reagent that can be considered to be the ideal or best protein assay method.
|
Protein Quantification Methods
|
Advantages
|
Disadvantages
|
|
Kjeldahl Method
|
Its universality, high precision and good reproducibility have made it the major method for the estimation of protein in foods.
|
It does not give a measure of the true protein, since all nitrogen in foods is not in the form of protein. Different proteins need different correction factors because they have different amino acid
sequences.
|
|
Colorometric Assays
|
Biuret method
|
The operation is simple, rapid, and less affected by the nature of protein species. It does not rely on amino acid composition and hence can measure all protein samples with accuracy.
|
The sensitivity is poor and the specificity is relative low. Buffers with Tris, Ammonia interferes with the reaction.
|
|
Lowry assay
|
High sensitivity, more specific and less interrupted by turbidity. It is very effective for the determination of water soluble protein content.
|
Time-consuming; susceptible to interferences such as K+, Mg2+, NH4+, EDTA, Tris-HCl, carbohydrates, and reducing agents.
|
|
BCA assay
|
The determination is quick and simple, sensitive and accurate; done in one step; not affected by chemical substances such as detergent in most samples. the reagent is fairly stable under alkaline
conditions and can be included in the copper solution to allow a one-step procedure
|
To increase assay sensitivity, the assay should be performed at an elevated temperature of 60 C. Compared to the bradford assay, the BCA assay is susceptible to interference by some chemicals
present in protein samples, including reducing agents (e.g. DTT and beta-mercaptoethanol), copper chelators (e.g. EDTA, EGTA), and buffers with high concentration.
|
|
Bradford Assay
|
Sensitive, rapid, convenient, widely used, simple operation, short reaction time, stable dye-protein color and strong anti-interference.
|
Major problems are the different response factors of proteins and a large number of interferences.
|
|
UV absorbance at 280 nm
|
Simple and easy to use, sensitive, fast, affordability and the sample is recoverable
|
Poor accuracy and specificity. Susceptible to interference from parallel substances, such as DNA.
|
|
ELISA
|
High throughput, high sensitivity and specificity, and convenient.
|
Relatively high cost.
|
5. How to Select An Appropriate Method for the Determination of Protein Concentration?
No single assay can be used to quantify all protein types. Because specific limitations of certain methods are required to be considered before selecting the appropriate
method that is most compatible with the sample. To ensure accurate measured data, several factors must be considered.
Choosing an appropriate method depends on the properties of proteins present in the samples as well as the characteristics of analytical approaches. In general, the choice of method for protein
concentration determination requires consideration of a number of factors, including interfering agents in the sample or lysis buffer, assay sensitivity and sample volume, sample preparation, speed of
analysis, convenience, and availability of reagents, assay time, protein standards, dilution of the protein sample, protein-to-protein variation, equipment requirements, as well as the compatibility with the
composition of the buffer of the sample to be analyzed.
6.Applications of Protein Quantification
Measurement of protein concentration is necessary for protein purification, electrophoresis, cell biology, molecular biology, and other research applications.
UV absorption method is widely used in the biochemical preparation of proteins and enzymes. This method is suitable for use with chromatographic columns for real-time monitoring of protein
adsorption or elution, but the results are not very accurate.
The Bradford method is suitable for large-scale sample analysis, however, samples should not include a high concentration of detergents, which necessitates more stringent sample standards. It is
recommended for general use, especially for assessing the protein content of cell fractions and estimating protein concentrations for gel electrophoresis.
The Lowry method is used in the measurement of protein during enzyme fractionations, mixed tissue proteins, measurement of very small absolute amounts of protein, or highly diluted protein and
analysis of large numbers of similar protein samples.
The standard BCA assay can be used to determine the protein concentrations of each homogenized sample.
In conclusion, although there are many methods to determine protein content, there is no perfect one for all experiments. In fact, one or more certain factors such as assay time, sensitivity, accuracy,
etc., that you attach importance to could help you quickly select an appropriate determination method. We hope this article will help those who are hesitant to choose a protein concentration assay.
References
[1] Lowry OH, Rose Brough NJ, et al. Protein measurement with the folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265-75.
[2] Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766.
[3] Smith PK, Krohn RI, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76-85.
[4] M M Bradford. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding [J]. Anal Biochem. 1976 May 7; 72:248-54.
[5] Sedmak JJ, Grossberg SE. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977;79:544–552.
[6] Simonian M. H. Spectrophotometric determination of protein concentration. In: Wrolstad R. E., editor. Handbook of Food Analytical Chemistry. New Jersey, NJ, USA: John Wiley & Sons; 2000. pp. 115
-121.
CUSABIO team. Approaches to Determinate Protein Concentration. https://www.cusabio.com/c-21049.html



Comments
Leave a Comment