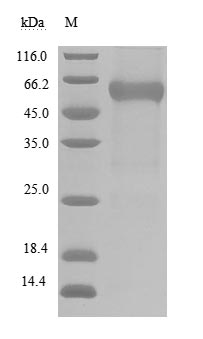
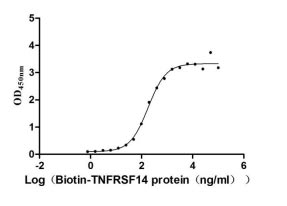
Producing recombinant human BTLA protein involves multiple steps: gene cloning, plasmid assembly, protein expression, purification, and evaluation. Specific primers amplify the gene fragment encoding amino acids 31-150 of the human BTLA, which is ligated into a plasmid carrying the C-terminal hFc-Myc-tag gene. Mammalian cells are transfected with the recombinant plasmid using a transfection reagent, followed by the addition of a selective antibiotic to screen the successfully transfected cells. The recombinant BTLA protein is released by lysing the cells and is purified from the supernatant using affinity chromatography. Its purity, confirmed via SDS-PAGE, exceeds 90%, and the LAL assay shows its endotoxin levels below 1.0 EU/μg. Functional ELISA confirms this active human BTLA protein binds the biotinylated human TNFRSF14 (CSB-MP842173HU-A) with an EC50 of 137.8-233.4 ng/mL.
The BTLA, also known as CD272, is an important co-inhibitory receptor belonging to the immunoglobulin superfamily. It plays a crucial role in the regulation of immune responses by negatively modulating the activity of T and B lymphocytes. BTLA is primarily expressed on various immune cells, including CD4+ and CD8+ T cells, B cells, natural killer (NK) cells, and antigen-presenting cells (APCs) such as dendritic cells and macrophages [1][2][3]. This widespread expression underscores its significance in maintaining immune homeostasis and preventing excessive immune activation that could lead to autoimmunity or tissue damage.
BTLA functions through its interaction with the herpesvirus entry mediator (HVEM), a member of the TNFR superfamily. The binding of BTLA to HVEM initiates intracellular signaling that inhibits T-cell activation, proliferation, and cytokine production, thereby dampening the immune response [3][4][5]. This mechanism is particularly relevant in the context of chronic infections and cancer, where BTLA can limit the effectiveness of immune responses against tumor cells or persistent pathogens [3][6][7].
Research has demonstrated that BTLA is involved in various pathological conditions, including autoimmune diseases and sepsis. Studies have shown that BTLA expression on CD4+ T cells is associated with sepsis and subsequent infections in critically ill patients, indicating its role in immunosuppression during severe illness [8][9]. Additionally, BTLA has been implicated in the development of autoimmune conditions, as evidenced by experiments in mice lacking BTLA, which developed autoimmune hepatitis-like disease [10][3].
References:
[1] L. Gherardi. Targeting b and t lymphocyte attenuator regulates lupus disease development in nzb/w mice,, 2024. https://doi.org/10.1101/2024.05.28.596218
[2] H. Douna, J. Amersfoort, F. Schaftenaar, I. Bot, C. Binder, H. Yagita, et al. Btla stimulation protects against atherosclerosis by regulating follicular b cells, Atherosclerosis, vol. 287, p. e100, 2019. https://doi.org/10.1016/j.atherosclerosis.2019.06.290
[3] A. Andrzejczak. Btla biology in cancer: from bench discoveries to clinical potentials, Biomarker Research, vol. 12, no. 1, 2024. https://doi.org/10.1186/s40364-024-00556-2
[4] M. Spodzieja, K. Kuncewicz, A. Sieradzan, A. Karczyńska, J. Iwaszkiewicz, V. Cesson, et al. Disulfide-linked peptides for blocking btla/hvem binding, International Journal of Molecular Sciences, vol. 21, no. 2, p. 636, 2020. https://doi.org/10.3390/ijms21020636
[5] M. Rio, C. Lucas, L. Bühler, G. Rayat, & J. Rodríguez-Barbosa. Hvem/light/btla/cd160 cosignaling pathways as targets for immune regulation, Journal of Leukocyte Biology, vol. 87, no. 2, p. 223-235, 2009. https://doi.org/10.1189/jlb.0809590
[6] Y. Chen, H. Lin, C. Chien, Y. Lai, W. Sun, C. Chen, et al. Btla blockade enhances cancer therapy by inhibiting il-6/il-10-induced cd19high b lymphocytes, Journal for Immunotherapy of Cancer, vol. 7, no. 1, 2019. https://doi.org/10.1186/s40425-019-0744-4
[7] S. Karakatsanis, G. Βertsias, P. Roussou, & D. Boumpas. Programmed death 1 and b and t lymphocyte attenuator immunoreceptors and their association with malignant t‐lymphoproliferative disorders: brief review, Hematological Oncology, vol. 32, no. 3, p. 113-119, 2013. https://doi.org/10.1002/hon.2098
[8] N. Shubin, S. Monaghan, D. Heffernan, C. Chung, & A. Ayala. B and t lymphocyte attenuator expression on cd4+ t-cells associates with sepsis and subsequent infections in icu patients, Critical Care, vol. 17, no. 6, 2013. https://doi.org/10.1186/cc13131
[9] E. Sherwood and R. Hotchkiss. Btla as a biomarker and mediator of sepsis-induced immunosuppression, Critical Care, vol. 17, no. 6, p. 1022, 2013. https://doi.org/10.1186/cc13143
[10] Y. Oya, N. Watanabe, T. Owada, M. Oki, K. Hirose, A. Suto, et al. Development of autoimmune hepatitis–like disease and production of autoantibodies to nuclear antigens in mice lacking b and t lymphocyte attenuator, Arthritis & Rheumatism, vol. 58, no. 8, p. 2498-2510, 2008. https://doi.org/10.1002/art.23674