Tumor necrosis factor receptor superfamily (TNFRSF) belongs to the transmembrane protein receptor, and its characteristic structure is its extracellular domain cysteine-rich domain (CRDs), which usually contains 6 cysteine residues involved in the formation of three disulfide bonds. The number of CRDs varies by receptor, from one to four. These Cys are ligand binding sites. TNFRSF can bind to the corresponding ligand and play an important role in inducing apoptosis, mediating inflammatory response and autoimmune diseases.
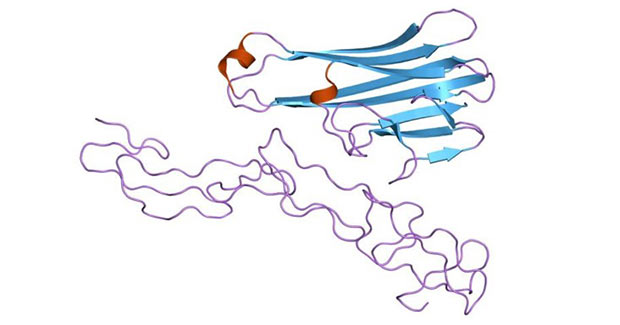
The image is from Wikipedia
Figure 1 Structure of the soluble human 55 kd TNF receptor-human TNF beta complex
1. Classification of TNFRSF
TNFRSF can be divided into two categories according to the presence or absence of Death domain in the membrane.
The first category mainly includes TNF-R1, Fas (Apo-1, CD95), DR3 (Apo-3, WSL-1, TRAMP), DR-4 (TRAIL-R1), DR-5 (TRAIL-R2), DR-6, EDA-R and NGFR.
Their intracellular region contains a death domain (DD) that is homologous to Drosophila cell death proteins. The death domain contains a receptor that transduces an apoptotic signaling pathway. When TNFR is activated by an apoptotic signal, its cytoplasmic death domain recruits apoptosis-related factors, which then activate downstream caspases to cause cellular apoptosis.
Members of the second subfamily include TNFR2, CD40, OX40, and 4-1BB. Although their cytoplasmic regions are quite different, they can regulate the activation of NF-kappa B(nuclear factor kappa B) or JNK(c-jun amino-terminal kinase) by mediating tumor necrosis factor receptor-related factors, thereby regulating gene expression and promoting growth [1].
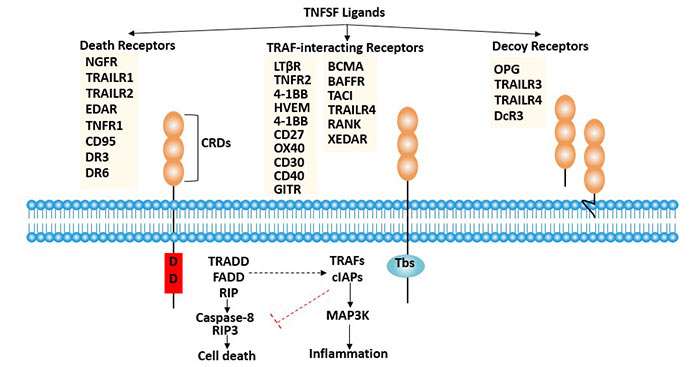
Figure 2 Member and classification of TNFRSF
3. Death Receptors
In TNFRSF, some receptor molecules have Death domain (DD) in the cytoplasmic region, which is also called Death receptor (DR). When these receptor molecules are combined with the corresponding ligands, a series of signaling molecules can be recruited and activated, eventually activating aspartic acid specific cysteine protease/Caspase in the cytoplasm, leading to apoptosis [4]. Therefore, death receptors are involved in many physiological and pathological processes of the body, especially in multiple viral infections.
3.1 Fas system
Fas exists widely in many tissues and cells, but is mainly expressed in immune cells.
Both Fas and FasL can be secreted or exfoliated outside the cell and become soluble functional molecules, namely sFas and sFasL. Fas, FasL, sFas and sFasL together form the Fas system.
FasL binds to Fas and causes apoptosis. The combination of sFasL and Fas also induces apoptosis. sFas has a high affinity with FasL, and can compete with membrane type Fas for FasL, but does not cause apoptosis, and indirectly inhibits apoptosis. The role of sFas and sFasL may be a regulatory mode of apoptosis in vivo. Apoptosis mediated by Fas system plays a very important role in transplantation immunity, which is involved in the development of lymphocytes, the formation of self-tolerance, the restriction of T lymphocyte cloning, the realization of immune response and many other immune processes.
3.2 TNFR1
Tumor necrosis factor receptor 1(TNFR1), also known as the tumor necrosis factor receptor superfamily member (TNFRSF1A) and CD120a, is a ubiquitous membrane receptor-binding tumor necrosis factor alpha (TNF alpha).
TNFR1 is expressed in almost every cell in the body. It is involved in apoptosis.
Compared with TNFR1, TNFR2 is expressed in limited cells, which determines that TNFR2 activity is more specific to disease and is a good therapeutic target [5]. TNFR2 is mainly dependent on tumor necrosis factor receptor-associated factor 2 (TRAF2) and nuclear factor-kappaB (NF-κB) in cell survival.
3.3 DR3
Death receptor 3(DR3) is a new member of the TNFR superfamily containing death domain discovered after tumor necrosis factor receptor (TNFR1) and Fas/ APO-l [6].
The DR3-encoded product is a type II transmembrane protein composed of an extracellular receptor binding region, a transmembrane region, and an intracellular death domain (DD). DR3 is mainly distributed in lymphoid tissues such as spleen, thymus and peripheral blood lymphocytes. The restricted expression pattern of DR3 in lymphoid tissue suggests that it may play a special role in the development and function of lymphocytes. DR3, like TNFR1 and Fas, can induce apoptosis and activate the nuclear factor kappa B.
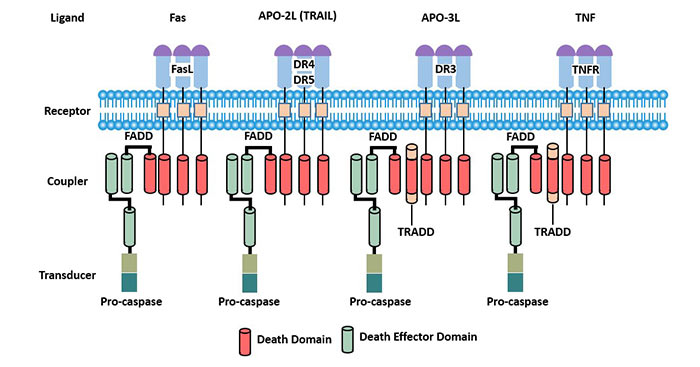
Figure 3 Death receptors
4. Other Receptors
4.1 TNFRSF19
Structure: TNFRSF19 (TROY) is a newly discovered tumor necrosis factor receptor superfamily expressed in mouse embryos, also known as toxicity and JNK inducer (TAJ). TROY is a type I transmembrane protein [7].
The extracellular region of TROY has a hydrophobic region as a signal peptide followed by two cysteine-rich regions and an incomplete cysteine region. Unlike the death receptor, the intracellular region of TROY does not have a death domain, but a TNF receptor-associated factor 2 (TRAF2) binding sequence.
Expression: TROY is widely expressed in a variety of tissues, but unlike most known TNFRSF members, TNFRSF19 is not expressed in major lymphoid tissues such as the spleen and thymus [8]. TROY is highly expressed in the embryonic and mature central nervous system. TNFRSF19 is highly expressed in the process of embryo development, indicating that TNFRSF19 gene plays a crucial role in embryo development.
TNFRSF19 gene expression is associated with many diseases, such as neuroblastoma, nasopharyngeal carcinoma, vascular dementia, etc. Abnormal expression of TNFRSF19 may be the driver of malignant glioma cell metastasis [9].
Function: In general, the tumor necrosis factor receptor (TNFR) superfamily induces apoptosis through the death domain. The intracellular domain of TROY has no death domain, which induces cell death in the form of para-apoptosis. Interaction of TNFRSF19 gene with TRAF family members can activate the JNK pathway and induce non-caspase-dependent cell death [10].
As an analogue of p75, TROY can form functional receptor complexes with NgR1 and LINGO-1 to mediate the inhibition signal of axon growth suppressor, thereby inhibiting axon growth.
TROY is involved in the development and differentiation of the nervous system. TROY may control the proliferation of cells and maintain the undifferentiated state of precursor cells during the development of the nervous system [11].
In addition, TROY may play an important role in cell proliferation. Studies have shown that TROY may play an important role in promoting cell growth, and its expression in melanoma cells may be one of the reasons for promoting tumor cell growth [12].
4.2 HVEM
Structure: HVEM, also known as TR2 (TNFR-related 2). It is a single transmembrane protein of 283 amino acid residues, including a 36AA signal peptide, a 164AA extracellular domain (containing four cysteine-rich regions, CRD1-4), a 24AA transmembrane region and a 59AA cytoplasmic zone.
Expression: TR2 mRNA is highly expressed in lung, spleen and thymus, and weakly expressed in bone marrow and endothelial cells, but not in brain, liver and skeletal muscle.
Biological effects: Four ligands of HVEM have been found, namely HSV-gD, LIGHT, LTα and BTLA. HVEM binding with different ligands plays different biological roles.
HSV-1 infects activated T cells, immature and mature DCs by binding to HVEM.
HVEM combined with LIGHT can exert a stimulating effect on T cells and B cells.
LIGHT and HVEM are newly discovered co-stimulatory signals for activation of T cells. LIGHT binds to its receptor HVEM/TR2 and has the functions of co-stimulating T lymphocytes and inducing apoptosis of tumor cells [13].
The HVEM-LIGHT signaling pathway plays an important role in transplantation immunity.
The HVEM-LIGHT signaling pathway is closely related to the development of inflammation and autoimmune diseases.
In addition, HVEM may also be involved in the formation of atherosclerotic plaques.
4.3 Decoy Receptor3 (DcR3)
Unlike most other TNFR superfamily members, DcR3 lacks a transmembrane structure and is a secreted protein.
Expression: DcR3 mRNA have low expression level in human embryo lung, brain, liver, stomach, spinal cord, lymph nodes, trachea, spleen and colon. However, the expression is increased in malignant tumors such as lung cancer, colorectal adenocarcinoma, esophageal cancer, gastric cancer, liver cancer, pancreatic cancer, EBV-related lymphoma and glioma, and autoimmune diseases such as systemic lupus erythematosus.
Function: DcR3 is an inhibitor of apoptosis protein. It competes with other receptors to bind to the ligands FasL, LIGHT and TLIA to inhibit apoptosis, thereby evading killing of killer cells. It can inhibit tumor cell apoptosis and promote tumor cell immune evasion.
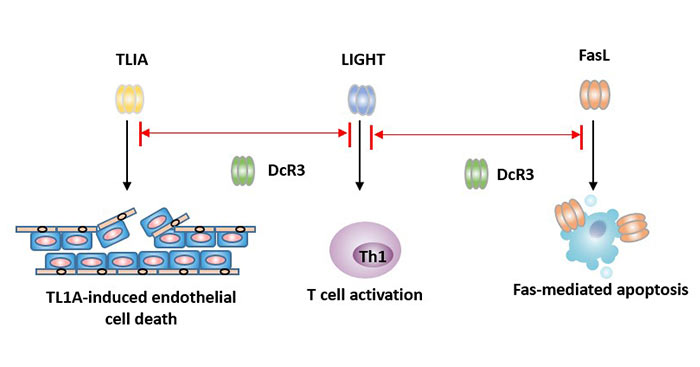
Figure 4 How does DcR3 inhibit apoptosis?
Based on its features, DcR3 has broad application prospects. It can be used as a new tumor marker for clinical diagnosis, efficacy observation and prognosis judgment. It provides a new way for tumor treatment at the immune and molecular levels. It is also used in the treatment of immune rejection after transplantation.
4.4 CD137
CD137 (4-1BB) is a type II membrane glycoprotein present on the surface of cell membranes. CD137 cDNA has a total length of 1.44kb and encodes a 255-amino acid protein of approximately 28kb. It contains extracellular regions, transmembrane hydrophobic regions, and intracellular regions.
CD137 is mainly expressed on the surface of T lymphocytes activated by phytohemagglutinin (PHA), phorbol ester (PMA) and CD3 mAb. In addition, CD137 is also expressed in some non-lymphocytes. However, CD137 was not expressed on the surface of stationary lymphocytes in human peripheral blood.
CD137 may play an important role in regulating lymphocyte proliferation and apoptosis. CD137 can be used as an immune checkpoint, and other members of the TNFRSF family are also important immune checkpoints.
4.5 OPG
Osteoprotegerin is a soluble secreted glycoprotein. It is synthesized in the cell as a monomer and secreted to the cell as a double body. Compared with other TNFR superfamily members, OPG lacks transmembrane and cytoplasmic regions, but contains seven major domains (D1-D7). The N-terminal contains four cysteine-rich regions (D1-D4), which are involved in ligand binding and are directly related to the inhibition of osseous cell (OC). D5 and D6 near the C-terminal are the homologous regions of the death domain, mediating cytotoxicity and transducing apoptosis signals of osteoclasts. D7 has a heparin binding site and a cysteine residue, which is necessary for the formation of disulfide bonds in homologous dimers.
It can inhibit OC differentiation, maturation, induce OC apoptosis and increase bone density, which is also the source of its name [14]. OPG competes with RANKL for binding to RANK (NF-κB receptor activator), thereby inhibiting the differentiation and maturation of osteoclasts and inducing osteoclast apoptosis. Osteoprotegerin is an important substance for osteoclast formation, differentiation and regulation. The study of osteoprotegerin has made great progress in osteoporosis, osteopetrosis, rheumatoid arthritis, bone tumor and cardiovascular disease.
4.6 RANK
RANK is a member of the tumor necrosis factor receptor superfamily, also known as tumor necrosis factor receptor superfamily (TNFRSF) 11a, which is a type I transmembrane protein molecule.
Expression: RANK is expressed in specific tissues and organs in vivo, such as bone, bone marrow, spleen, lung, kidney, small intestine, and thymus.
Function: RANK is found to be highly expressed in breast cancers with more bone metastases [15] and prostate cancer cells, and is a poor prognostic factor for cancer patients with bone metastases. It also promotes the proliferation of cervical cancer cells HeLa and SiHa [16], indicating its potential to promote the development of cervical cancer.
References
[1] Arch R H, Gedrich R W, Thompson C B. Tumor necrosis factor receptor-associated factors (TRAFs)-a family of adapter proteins that regulates life and death [J]. Genes & Development, 1998, 12(18): 2821-2830.
[2] Locksley R M, Killeen N, Lenardo M J. The TNF and TNF receptor superfamilies: Integrating mammalian biology [J]. Cell, 2001, 104(4): 487-501.
[3] Kwon B S, Tan K B, Ni J, et al. A Newly Identified Member of the Tumor Necrosis Factor Receptor Superfamily with a Wide Tissue Distribution and Involvement in Lymphocyte Activation *[J]. Journal of Biological Chemistry, 1997, 272.
[4] Schneider P, Tschopp J. Modulation of death receptor signaling [J]. Symposia of the Society for Experimental Biology, 2000, 52: 31.
[5] Faustman D L, Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine [J]. Frontiers in Immunology, 2013, 4.
[6] Daniel P T, Wieder T, Sturm I, et al. The kiss of death: promises and failures of death receptors and ligands in cancer therapy [J]. Leukemia (Basingstoke), 2001, 15(7): 1022-1032.
[7] Kojima T, Morikawa Y, Copeland N G, et al. TROY, a Newly Identified Member of the Tumor Necrosis Factor Receptor Superfamily, Exhibits a Homology with Edar and Is Expressed in Embryonic Skin and Hair Follicles [J]. Journal of Biological Chemistry, 2000, 275(27): 20742-20747.
[8] Hu S, Tamada K, Ni J, et al. Characterization of TNFRSF19, a Novel Member of the Tumor Necrosis Factor Receptor Superfamily[J]. Genomics, 1999, 62(1): 0-107.
[9] Paulino V M, Yang Z, Kloss J, et al. TROY (TNFRSF19) Is Overexpressed in Advanced Glial Tumors and Promotes Glioblastoma Cell Invasion via Pyk2-Rac1 Signaling [J]. Molecular Cancer Research, 2010, 8(11): 1558-1567.
[10] Eby M T. TAJ, a Novel Member of the Tumor Necrosis Factor Receptor Family, Activates the c-Jun N-terminal Kinase Pathway and Mediates Caspase-independent Cell Death [J]. Journal of Biological Chemistry, 2000, 275(20): 15336-15342.
[11] Hisaoka T, Morikawa Y, Komori T, et al. Characterization of TROY-expressing cells in the developing and postnatal CNS: the possible role in neuronal and glial cell development [J]. European Journal of Neuroscience, 2006, 23(12).
[12] Spanjaard R A, Whren K M, Graves C, et al. Tumor necrosis factor receptor superfamily member TROY is a novel melanoma biomarker and potential therapeutic target [J]. International Journal of Cancer, 2007, 120(6): 1304-1310.
[13] Fan Z, Yu P, Wang Y, et al. NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors [J]. Blood, 2006, 107(4): 1342-1351.
[14] Boyce B F, Xing L. Biology of RANK, RANKL, and osteoprotegerin [J]. Arthritis Research & Therapy, 2007, 9.
[15] Zhang L, Teng Y, Zhang Y, et al. Receptor activator for nuclear factor κB expression predicts poor prognosis in breast cancer patients with bone metastasis but not in patients with visceral metastasis [J]. Journal of Clinical Pathology, 2012, 65(1): 36-40.
[16] Shang W Q, Li H, Liu L B, et al. RANKL/RANK interaction promotes the growth of cervical cancer cells by strengthening the dialogue between cervical cancer cells and regulation of IL-8 secretion [J]. Oncology Reports, 2015, 34(6): 3007.
[17] Ye Q. Modulation of LIGHT-HVEM Costimulation Prolongs Cardiac Allograft Survival [J]. Journal of Experimental Medicine, 2002, 195(6): 795-800.







Comments
Leave a Comment