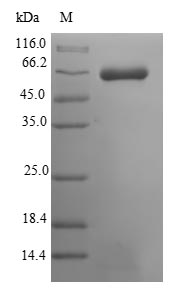
To produce recombinant mouse Muscle, skeletal receptor tyrosine-protein kinase (Musk) in E. coli, the gene encoding the extracellular domain of the mouse Musk protein (22-494aa) is co-cloned into an expression vector with an N-terminal 10xHis-SUMO-tag and C-terminal Myc-tag gene and transformed into E. coli cells. The bacteria are cultured under conditions that induce protein expression. Once adequate growth is achieved, the cells are lysed to release the recombinant Musk protein. The recombinant Musk protein is subjected to affinity chromatography purification. Its purity is confirmed using SDS-PAGE, greater than 90%.
MuSK is a receptor tyrosine kinase expressed in skeletal muscle [1][2][3]. MuSK is involved in the neuromuscular junction (NMJ) formation and maintenance [4][5]. Agrin, a ligand derived from motor neurons, interacts with MuSK to stimulate its phosphorylation, a process critical for synapse formation [6][7]. MuSK is involved in acetylcholine receptor clustering at the neuromuscular junction, a process necessary for proper synaptic function [8][9].
Studies have shown that MuSK is expressed early in synaptogenesis and is vital for the development and maintenance of the NMJ [2][10]. MuSK has been linked to cholinergic responses, synaptic plasticity, and memory formation in the brain [11]. The interaction of MuSK with other proteins like Src-class kinases and 14-3-3γ further highlights its role in regulating synaptic gene transcription and acetylcholine receptor phosphorylation [5][12].
References:
[1] N. Kim, A. Stiegler, T. Cameron, P. Hallock, A. Gomez, J. Huanget al., Lrp4 is a receptor for agrin and forms a complex with musk, Cell, vol. 135, no. 2, p. 334-342, 2008. https://doi.org/10.1016/j.cell.2008.10.002
[2] D. Reuveni, R. Aricha, M. Souroujon, & S. Fuchs, Musk eamg: immunological characterization and suppression by induction of oral tolerance, Frontiers in Immunology, vol. 11, 2020. https://doi.org/10.3389/fimmu.2020.00403
[3] A. Yilmaz, C. Kattamuri, R. Özdeşlik, C. Schmiedel, S. Mentzer, C. Schorlet al., Musk is a bmp co-receptor that shapes bmp responses and calcium signaling in muscle cells, Science Signaling, vol. 9, no. 444, 2016. https://doi.org/10.1126/scisignal.aaf0890
[4] S. Hubbard and K. Gnanasambandan, Structure and activation of musk, a receptor tyrosine kinase central to neuromuscular junction formation, Biochimica Et Biophysica Acta (Bba) - Proteins and Proteomics, vol. 1834, no. 10, p. 2166-2169, 2013. https://doi.org/10.1016/j.bbapap.2013.02.034
[5] L. Strochlic, A. Cartaud, A. Méjat, R. Grailhe, L. Schaeffer, J. Changeuxet al., 14-3-3 γ associates with muscle specific kinase and regulates synaptic gene transcription at vertebrate neuromuscular synapse, Proceedings of the National Academy of Sciences, vol. 101, no. 52, p. 18189-18194, 2004. https://doi.org/10.1073/pnas.0406905102
[6] Y. Zong, B. Zhang, S. Gu, K. Lee, J. Zhou, G. Yaoet al., Structural basis of agrin–lrp4–musk signaling, Genes & Development, vol. 26, no. 3, p. 247-258, 2012. https://doi.org/10.1101/gad.180885.111
[7] P. Hallock, C. Xu, T. Park, T. Neubert, T. Curran, & S. Burden, Dok-7 regulates neuromuscular synapse formation by recruiting crk and crk-l, Genes & Development, vol. 24, no. 21, p. 2451-2461, 2010. https://doi.org/10.1101/gad.1977710
[8] A. Fu, F. Smith, H. Zhou, A. Chu, K. Tsim, B. Penget al., xenopus muscle‐specific kinase: molecular cloning and prominent expression in neural tissues during early embryonic development, European Journal of Neuroscience, vol. 11, no. 2, p. 373-382, 1999. https://doi.org/10.1046/j.1460-9568.1999.00443.x
[9] C. Fuhrer, M. Gautam, J. Sugiyama, & Z. Hall, Roles of rapsyn and agrin in interaction of postsynaptic proteins with acetylcholine receptors, Journal of Neuroscience, vol. 19, no. 15, p. 6405-6416, 1999. https://doi.org/10.1523/jneurosci.19-15-06405.1999
[10] A. Watty, G. Neubauer, M. Dreger, M. Zimmer, M. Wilm, & S. Burden, The in vitro and in vivo phosphotyrosine map of activated musk, Proceedings of the National Academy of Sciences, vol. 97, no. 9, p. 4585-4590, 2000. https://doi.org/10.1073/pnas.080061997
[11] A. García‐Osta, P. Tsokas, G. Pollonini, E. Landau, R. Blitzer, & C. Alberini, Musk expressed in the brain mediates cholinergic responses, synaptic plasticity, and memory formation, Journal of Neuroscience, vol. 26, no. 30, p. 7919-7932, 2006. https://doi.org/10.1523/jneurosci.1674-06.2006
[12] A. Mohamed, K. Rivas-Plata, J. Kraas, S. Saleh, & S. Swope, Src-class kinases act within the agrin/musk pathway to regulate acetylcholine receptor phosphorylation, cytoskeletal anchoring, and clustering, Journal of Neuroscience, vol. 21, no. 11, p. 3806-3818, 2001. https://doi.org/10.1523/jneurosci.21-11-03806.2001