ROR1 has emerged as a key target, with multiple pharmaceutical companies advancing ROR1-targeted drugs into clinical trials. Recently, Merck & Co. acquired Zilovertamab Vedotin (MK-2140) for $2.75 billion, and its clinical trial application has been accepted again. MK-2140, a drug combining a humanized monoclonal antibody UC-961 with the microtubule inhibitor MMAE, targets ROR1-positive tumor cells and releases cytotoxic agents to inhibit tumor growth, showing promising results in clinical trials for hematological malignancies. Similarly, CSPC Pharmaceutical Group's ROR1-targeted ADC drug SYS6005 has received approval from China's National Medical Products Administration (NMPA) for clinical trials, indicating its potential for treating advanced solid tumors. These developments highlight the growing interest and potential of ROR1-targeted drugs in cancer therapy, suggesting they may offer new treatment options for cancer patients in the future [1-2].
1. What is ROR1?
ROR1 is a member of the receptor tyrosine kinase-like orphan receptor (ROR) family, which belongs to the receptor tyrosine kinase (RTK) family. Unlike the ROR family of nuclear receptors, ROR is a type of membrane receptor. They are closely related to the tropomyosin-related kinase receptor family (tropomyosin-related kinase, Trk), skeletal muscle specific tyrosine kinase-like receptor family (muscle specific knase, MuSK) and neurotrophic factor esterine kinase receptor family (NT-RTK). Since the ligand of the ROR protein was unknown when it was first discovered, it was defined as an orphan receptor [3].
ROR2 is another member of the ROR family, and the amino acid sequence homology between ROR1 and ROR2 is 58%. Among them, ROR1 contains two subtypes: complete cell membrane receptor type ROR1 and truncated variants. Truncated variants are mainly divided into two subtypes, including membrane-bound ROR1I without extracellular structure and soluble ROR1 with only extracellular structure [4]. In fact, there is no difference in the expression of soluble ROR1 between normal people and cancer patients. Moreover, the expression in serum is very low or undetectable, and has nothing to do with the progression or severity. However, the intact membrane-bound ROR1 is specifically expressed at a high level in a variety of tumor tissues [5]. Note that the ROR1 mentioned in the following all refers to the membrane-bound ROR1 with a complete structure.
2. The Structure of ROR1
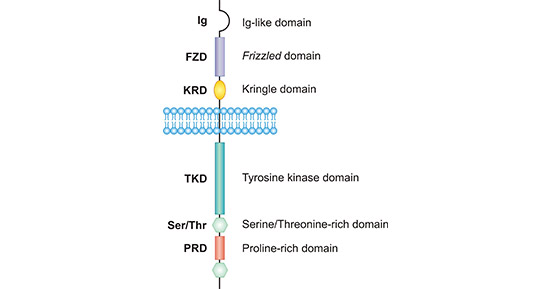
ROR1 is a transmembrane receptor tyrosine kinase protein. The gene of ROR1 is located on chromosome 1p31.3 with a length of 2814bp. The protein encoded by ROR1 gene is composed of 937 amino acids and has a molecular weight of about 105 kDa. The structure of ROR1 is highly conserved among biological species, such as human and mouse ROR1 amino acid sequence homology can reach 97%. As shown in Figure 1, human ROR1 consists of an extracellular immunoglobulin-like domain (Ig), two cysteine-rich domains (FZD), a proximal membrane kringle domain, and a single transmembrane structure. On the intracellular side, ROR1 possesses an intracellular tyrosine kinase domain (TKD), two serine/threonine enrichment domains (S/TRD) and a proline enrichment domain (PRD) composed [6].
Figure 1. The structure of ROR1
*This figure is derived from the publication on Protein Cell [6]
3. The Function of ROR1
The immunoglobulin-like domain and cysteine-rich domain are related to the function of ROR1 binding ligand. The FZD domain consists of 10 conserved cysteine residues and 5 corresponding disulfide bonds. FZD structures are also found in smooth muscle family receptors (such as Smo), frizzled family receptors (such as sFRP), as well as carboxypeptidase Z (CPZ), collagen α1 XVIII and low-density lipoprotein receptor-related protein (LRP). Most of these proteins can bind to Wnt protein and participate in signal transduction of the Wnt pathway.
Currently, a large number of studies have confirmed that ROR1 also binds Wnt5a through FZD and activates the non-canonical Wnt signaling pathway [7] [8]. The Kringle domain is relatively conserved in the ROR family and consists of 80 amino acids [9]. Its structural feature is that it contains three ring structures formed by disulfide bonds, and these three ring structures are also involved in the process of ROR receptor recognition of Wnt protein.
The tyrosine kinase domain of ROR is highly conserved in organisms and is very similar to the tyrosine kinase domains of Trk and MuSK. But ROR1 protein has some amino acid sequence changes in this highly conserved region, suggesting that its intracellular kinase activity may change. The serine/threonine and proline-rich domains are composed of serine/threonine-rich domains, proline-rich domains, and serine/threonine-rich domains in series. There are potential phosphorylation sites in this region, which is related to downstream signal transduction.
4. Wnt5a/ROR1 and Tumor
At present, in terms of ROR1 signaling, mainstream evidence is that ROR1 can play an important role in a variety of physiological processes by mediating non-canonical Wnt signaling pathways, including regulating cell division, proliferation, migration, and cell chemotaxis, especially binding to Wnt5a. Wnt5a is a typical activator of non-canonical Wnt signaling pathway. It participates in the phosphorylation of NF-κB subunit p65, activates the NF-κB signaling pathway in tumor cells, promotes cell migration and invasion, EMT, cancer metastasis, etc. Next, let's learn more about how ROR1 activates tumor cells through the Wnt5a signaling pathway.
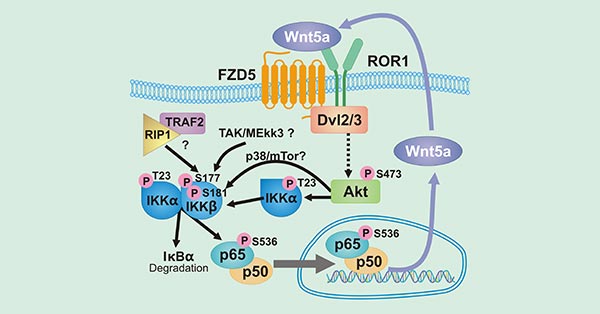
Wnt5a/ROR1 is highly expressed in various cancers. As the receptor of Wnt5a, ROR1 is involved in activating the NF-κB pathway of tumor cells. The NF-κB pathway is the helm of inflammation and immune regulation, and is constitutively activated in a variety of tumor types. As shown in Figure 2, Wnt5a activates the receptor ROR1 or FZD5, leading to Dv12/3 activation and Akt phosphorylation. Then, phosphorylated Akt promotes phosphorylation of IKKα to activate the IKK complex, which is responsible for the degradation of IκBα and the phosphorylating NF-κB subunit p65. And phosphorylated p65 transfers to the nucleus, promoting transcriptional expression of target genes including Wnt5a. The secretion of Wnt5a promotes a new round of autonomous feedback loop. The activation of the autonomous feedback loop ROR1/Akt/p65 pathway will further promote the secretion of pro-inflammatory factors (such as IL-6) and chemokines (such as CCL2).
Figure 2. The diagram of Wnt5a/ROR1 signaling pathway
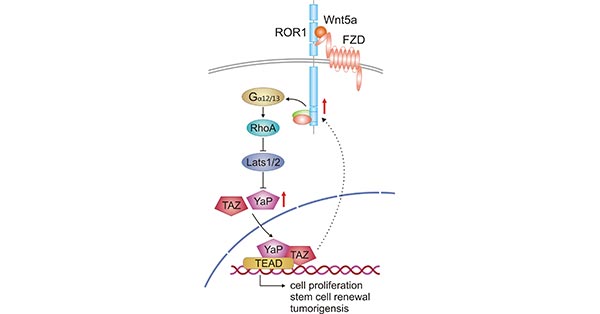
In addition, many studies have associated the expression of ROR1 with the activation of YAP/TAZ transcription, thereby enhancing tumorigenesis and chemoresistance [10] [11]. As shown in Figure 3, the binding of Wnt5a to the ROR1/FZD complex then further activates RhoA through binding to Gα12/13, thereby inhibiting Lats1/2 activity, leading to YAP/TAZ dephosphorylation and nuclear translocation. The YAP/TAZ translocated into the cell nucleus combined with TEAD can induce the transcription of genes involved in cell proliferation, stem cell self-renewal and tumorigenesis. Increased YAP/TAZ transcription can in turn up-regulate ROR1 expression [12].
Figure 3. Crosstalk between Wnt5a-ROR1 signaling and YAP/TAZ signaling pathway
*This figure is derived from the publication on Cells [12]
5. ROR1 Clinical Research Prospects
ROR1-targeted drugs are rapidly developing, with over 90 clinical candidates currently in research, with some already in Phase 3 clinical trials, indicating that ROR1-targeted drugs have the potential to be approved and marketed in the coming years, providing new treatment options for cancer patients. These drugs cover various types and mechanisms, including CAR-T cell therapies, ADC drugs, and monoclonal antibodies (Table 1).
CAR-T drugs, such as ONCT-808 and JCAR024, use genetic engineering to modify patients' T cells so that they express chimeric antigen receptors (CARs) specific to ROR1. When these CAR-T cells are infused back into the patient, they can precisely recognize and bind to ROR1 on the surface of tumor cells, activating the T cells' killing function and directly attacking the tumor cells, leading to their apoptosis.
ADC drugs, like MK-2140, consist of a humanized IgG1 monoclonal antibody targeting ROR1, a cleavable linker, and a toxic payload (such as monomethyl auristatin E, MMAE). The drug first binds specifically to ROR1 on the surface of tumor cells through the antibody component, is then internalized by the tumor cell, and releases the toxic payload inside the cell. The payload, MMAE, acts as a microtubule inhibitor, disrupting the microtubule function of the tumor cell and inhibiting mitosis, ultimately leading to cell death.
Monoclonal antibodies, such as ZILO-301 (Zilovertamab), are humanized monoclonal antibodies that specifically target ROR1. They can precisely recognize and bind to the ROR1 receptor on the surface of tumor cells, potentially triggering the body's immune response. For example, they can activate the complement system to induce complement-dependent cytotoxicity (CDC) or engage in antibody-dependent cell-mediated cytotoxicity (ADCC), where immune cells (such as natural killer cells) recognize and kill the tumor cells.
| Drug name |
Target |
Drug type |
Indications |
Institutions |
Highest Clinical phase |
| Zilovertamab |
ROR1 |
monoclonal antibody |
Mantle cell lymphoma |
University of California San Diego | University of California | Oncternal Therapeutics, Inc. |
Clinical stage 3 |
| Zilovertamab vedotin |
ROR1 x Tubulin |
ADC |
Diffuse large B-cell lymphoma | recurrent diffuse large B-cell lymphoma | B-cell lymphoma | B-cell malignant tumor | chronic lymphocytic leukemia | follicular lymphoma | mantle cell lymphoma | refractory diffuse large B-cell lymphoma | disseminated diffuse large B-cell lymphoma | bladder cancer | Burkitt's lymphoma | Ewing's sarcoma | neuroblastoma | Precursor B cell acute lymphoblastic leukemia | solid tumor | acute lymphoblastic leukemia | acute myeloid leukemia | marginal zone B cell lymphoma | T cell lymphoma | macroglobulinemia |
Merck Sharp & Dohme Corp. | Merck Sharp & Dohme LLC | Merck & Co., Inc. |
Clinical stage 2/3 |
| CAR T-cell therapy(Peking University) |
ROR1 |
Autologous CAR-T |
Advanced malignant solid tumor |
China people' s liberation army joint logistics support unit of the 92nd hospital | Peking University |
Clinical stage 1/2 |
| ROR1 CAR-T (Oncternal Therapeutics) |
ROR1 |
Autologous CAR-T |
Non-hodgkin's lymphoma |
Oncternal Therapeutics, Inc. |
Clinical stage 1/2 |
| EMB-07 |
CD3 x ROR1 |
Bispecific t cell conjugate |
Refractory lymphoma | lung adenocarcinoma | bladder cancer | breast cancer | uterine cancer |
An mai biological technology (suzhou) co ., ltd |
Clinical stage 1 |
| HDM-2005 |
ROR1 |
ADC |
Advanced malignant solid tumor |
Hangzhou Sino-American East China Pharmaceutical Co., Ltd. |
Clinical stage 1 |
| JCAR-024 |
ROR1 |
Autologous CAR-T |
Blood tumor |
Sino-American Shanghai Squibb Pharmaceutical Co., Ltd. |
Clinical stage 1 |
| LCB-71 |
ROR1 |
ADC |
Acute lymphoblastic leukemia |
Liga Chem Biosciences, Inc. | ABL Bio, Inc. | Wuxi Yaoming Helian Biotechnology Co., Ltd. | Qishi Pharmaceutical (Suzhou) Co., Ltd. |
Clinical stage 1 |
| LYL-797 |
ROR1 |
CAR-T |
Advanced breast cancer |
Lyell Immunopharma, Inc. |
Clinical stage 1 |
| NVG-111 |
CD3 x ROR1 |
Bispecific t cell conjugate |
Chronic lymphocytic leukemia |
Novalgen Ltd. |
Clinical stage 1 |
| PRGN 3007 |
ROR1 |
Autologous CAR-T |
Acute lymphoblastic leukemia |
Precigen, Inc. | Precigen ActoBio, Inc. |
Clinical stage 1 |
| ROR1-CAR-TILs(Guangdong Zhaotai InVivo Biomedicine) |
HPK1 x PD-1 x ROR1 |
CAR-TILs |
Advanced malignant solid tumor |
Guangdong zhaotai in vivo biological medicine technology co., ltd |
Clinical stage 1 |
| ROR1R-CAR(The University of Texas MD Anderson Cancer Center) |
ROR1 |
CAR-T |
leukemia |
The University of Texas MD Anderson Cancer Center | CLL Global Research Foundation |
Clinical stage 1 |
| SYS-6005 |
ROR1 |
ADC |
Solid tumor |
Unacon Jushi Biological Pharmaceutical Co., Ltd. |
Clinical application |
| Anti-ROR1 antibody scFv conjugates(Oncternal) |
ROR1 |
ADC |
tumour |
University of California San Diego | Oncternal Therapeutics, Inc. |
Drug discovery |
| CN114539411 |
ROR1 |
monoclonal antibody |
tumour |
Shandong boan biotechnology co ., ltd |
Drug discovery |
| CN116410317 |
ROR1 |
ADC |
Hyperplasia |
Sichuan Kelun Botai Biological Medicine Co., Ltd. |
Drug discovery |
| NVG-333 |
ROR1 |
Autologous CAR-T |
tumour |
Novalgen Ltd. |
Drug discovery |
| ROR1 (biological research) |
ROR1 |
CAR-T |
Chronic lymphocytic leukemia |
Zhejiang shengyan biological technology co ., ltd |
Drug discovery |
Table 1. Part ROR1 clinical drug development
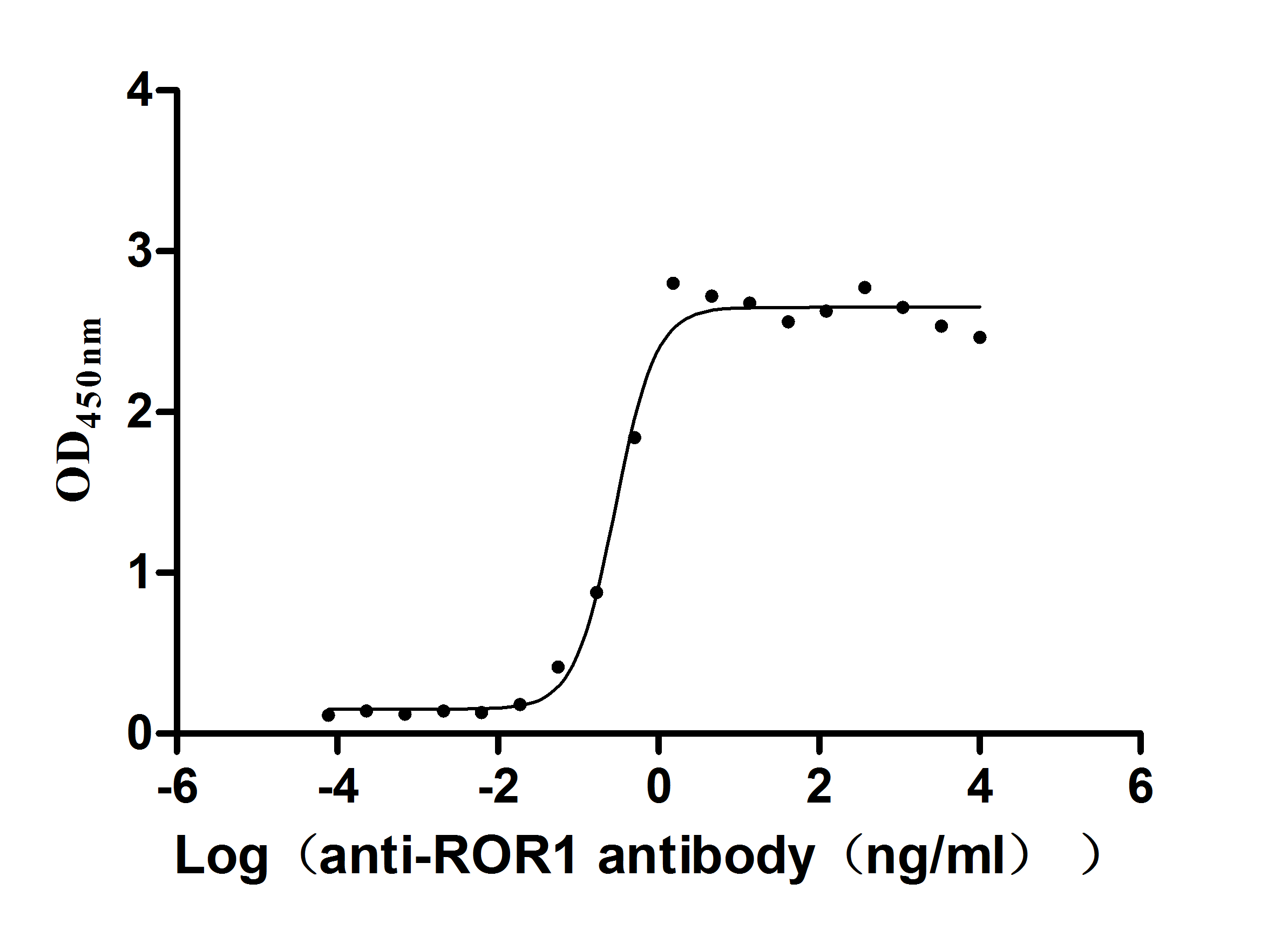
To fully support pharmaceutical companies in the ROR1-targeted drug research for leukemia and tumor, CUSABIO presents ROR1 active protein products (Code: CSB-MP020067HU1d7) to assist you in your research on the mechanism of ROR1 or its potential clinical value.
References
[1] https://www.nasdaq.com/press-release/merck-data-ash-2024-annual-meeting-highlights-promising-hematology-pipeline-diverse
[2] https://xueqiu.com/9353448186/307854678
[3] Forrester WC. The Ror receptor tyrosine Kinase family [J]. CMLS Cell Mol Life Sci. 2002, 59:83-06.
[4] Boreherding N, Kusner D, Liu GH, et al. ROR1, an embryonic protein with an emerging role in cancer biology [J]. Protein Cell. 2014, 5:496-502.
[5] Baskar S, K wong KY, .Hofer T, et al. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia [J]. Clin Cancer Res. 2008, 14:396-404.
[6] Nicholas Borcherding, David Kusner, Guang-Hui Liu, et al. ROR1, an embryonic protein with an emerging role in cancer biology [J]. Protein Cell. 2014, 5(7):496–502.
[7] Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin–TCF signaling depending on receptor context [J]. PLoS Biol. 2006, 4:e115.
[8] Nomi M, Oishi I, Kani S, et al. Loss of mRor1 enhances the heart and skeletal abnormalities in mRor2-deficient mice: redundant and pleiotropic functions of mRor1 and mRor2 receptor tyrosine kinases [J]. Mol Cell Biol. 2001, 21:8329–8335.
[9] Wamg HY, Lie T, Malbon CC, et al. Structure-function analysis of Frizzleds [J]. Cell Signal. 2006, 18: 934-941.
[10] Zhang, S., Zhang, H., Ghia, E.M., et al. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody [J]. Proc. Natl. Acad. Sci. USA. 2019, 116, 1370–1377.
[11] Islam, S.S., Uddin, M., Noman, A.S.M., et al. Antibody-drug conjugate T-DM1 treatment for HER2+ breast cancer induces ROR1 and confers resistance through activation of Hippo transcriptional coactivator YAP1 [J]. EBioMedicine. 2019, 43, 211–224.
[12] Hanna Karvonen, Harlan Barker, Laura Kaleva, et al. Molecular Mechanisms Associated with ROR1-Mediated Drug Resistance: Crosstalk with Hippo-YAP/TAZ and BMI-1 Pathways [J]. Cells. 2019, 8, 812: 2-12.
CUSABIO team. ROR1, an Emerging Target for Tumor Immunotherapy. https://www.cusabio.com/c-21068.html




Comments
Leave a Comment