Multicellular organisms live in a complex milieu where signaling pathways play a key role in their survival. As important mediators of this signal cascade, tyrosine kinases exert critical effects in various
biological processes such as growth, differentiation, metabolism, and apoptosis in response to internal and external stimuli. Among the 90 unique tyrosine kinase genes identified in the human genome, 58
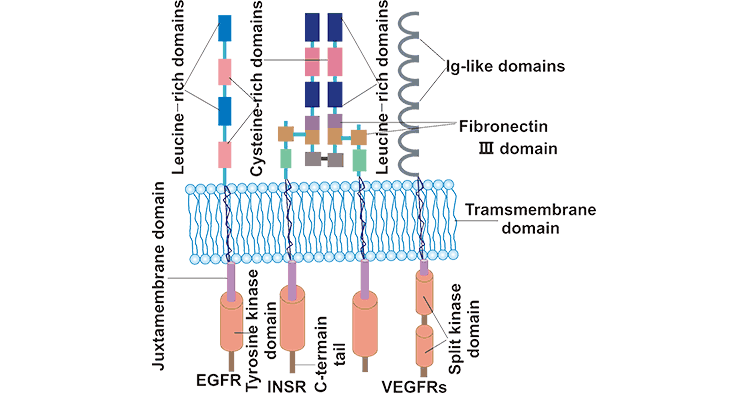
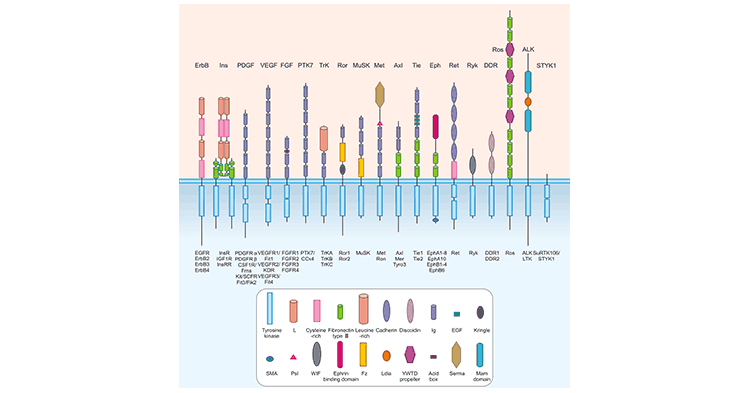
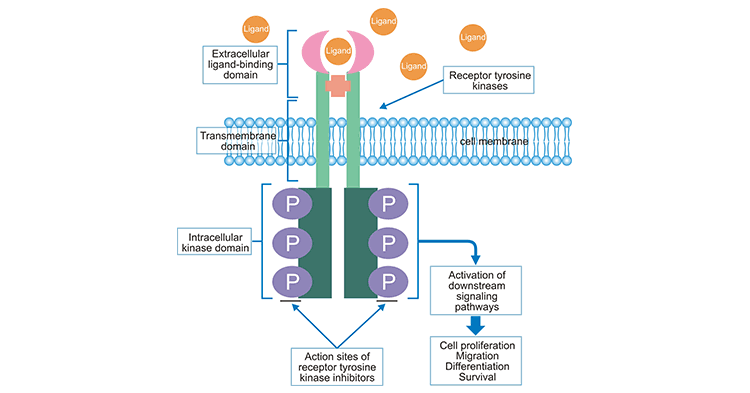
encode receptor tyrosine kinases (RTKs) [1] [2]. Here we provide a brief overview of the RTK superfamily, classification, functions, their regulatory mechanisms in cells, and related
diseases.
References
[1] D R Robinson, Y M Wu, et al. The protein tyrosine kinase family of the human genome [J]. Oncogene. 2000 Nov 20; 19(49):5548-57.
[2] Manning G, Plowman GD, et al. Evolution of protein kinase signaling from yeast to man [J]. Trends Biochem Sci 2002, 27:514–520.
[3] Hunter T. The Croonian lecture, 1997. The phosphorylation of proteins on tyrosine its role in cell growth and disease [J]. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1998; 353: 583-605.
[4] Hubbard SR. Structural analysis of receptor tyrosine kinases [J]. Prog Biophys Mol Biol. 1999;71:343–58.
[5] Li E, Hristova K. Receptor tyrosine kinase transmembrane domains: function, dimer structure and dimerization energetics [J]. Cell Adhes Migr 2010, 4(2):249–254.
[6] Manning G, Whyte DB, et al. The protein kinase complement of the human genome [J]. Science. 2002;298:1912–34.
[7] Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine [J]. Genes Dev. 2008;22:1276–1312.
[8] Reichardt LF. Neurotrophin-regulated signalling pathways [J]. Philos Trans R Soc Lond B Biol Sci 2006, 361: 1545–1564.
[9] Zhang, Y., Xia, M., Jin, K. et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities [J]. Mol Cancer 17, 45 (2018).
[10] Lyu J, Yamamoto V, Lu W. Cleavage of the Wnt receptor Ryk regulates neuronal differentiation during cortical neurogenesis [J]. Dev Cell 2008, 15: 773–780.
[11] Zhenfang Du & Christine M. Lovly. Mechanisms of receptor tyrosine kinase activation in cancer [J]. Molecular Cancer volume 17, Article number: 58 (2018).
[12] Christine Chiasson-MacKenzie and Andrea I McClatchey. Cell-Cell Contact and Receptor Tyrosine Kinase Signaling [J]. Cold Spring Harb Perspect Biol. 2018 Jun 1;10(6):a029215.
[13] Du Y., Hsu J.L., et al. Nuclear Functions of Receptor Tyrosine Kinases. In: Wheeler D., Yarden Y. (eds) Receptor Tyrosine Kinases: Structure, Functions and Role in Human Disease [J].
Springer, New York, NY. 2015.
[14] Fantl WJ, Johnson DE, Williams LT. Signaling by Receptor Tyrosine Kinases [J]. Annu Rev Biochem. 1993;62:453–481.
[15] Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases [J]. Cell. 2010;141:1117–34.
[16] Huse M and Kuriyan J. The conformational plasticity of protein kinases [J]. Cell, 2002, 109:275–282.
[17] Schlessinger J. Cell signaling by receptor tyrosine kinase [J]s. Cell. 2000;103:211–25.
[18] Lu Z., Jiang G., Jensen P., Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase [J]. Mol Cell
Biol. 2001;21:4016–4031.
[19] Bose R., Kavuri S.M., et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer [J]. Cancer Discov. 2013;3:224–237.
[20] Nakajima H., Ishikawa Y., et al. Protein expression, gene amplification, and mutational analysis of EGFR in triple-negative breast cancer [J]. Breast Cancer. 2014;21:66–74.
[21] Lynch T.J., Bell D.W., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib [J]. N Engl J Med.
2004;350:2129–2139.
[22] Peraldo-Neia C., Migliardi G., et al. Epidermal growth factor receptor (EGFR) mutation analysis, gene expression profiling and EGFR protein expression in primary prostate cancer [J]. BMC
Cancer. 2011;11:31.
[23] Fu M., Zhang W., et al. Mutation status of somatic EGFR and KRAS genes in Chinese patients with prostate cancer (PCa) Virchows Arch [J]. 2014;464:575–581.
[24] Joseph Schlessinger. Receptor Tyrosine Kinases: Legacy of the First Two Decades [J]. Cold Spring Harb Perspect Biol. 2014 Mar; 6(3): a008912.
[25] Tsou WI, Nguyen KQ, et al. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation [J]. J Biol Chem 2014, 289:
25750–25763.
[26] Lemke G. Biology of the TAM receptors [J]. Cold Spring Harb Perspect Biol2013, 5: a009076.
[27] Thayer K. Darling and Tracey J. Lamb. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity [J]. Front. Immunol., 04 July 2019.
[28] Vogel, W. F., Aszódi, A., et al. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development [J]. Mol. Cell. Biol. 2001, 21, 2906–2917.
[29]Labrador, J. P., Azcoitia, V., Tuckermann, et al. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism [J]. EMBO Rep.2001, 2, 446–452.
[30] Kamizaki K, Endo M, et al. Role of Noncanonical Wnt Ligands and Ror-family Receptor Tyrosine Kinases in the Development, Regeneration, and Diseases of the Musculoskeletal System [J].
Dev Dyn (2021) 250(1):27–38.
[31] Stevan R. Hubbard and Kavitha Gnanasambandan. Structure and Activation of MuSK, a Receptor Tyrosine Kinase Central to Neuromuscular Junction Formation [J]. Biochim Biophys Acta. 2013 Oct;
1834(10): 2166–2169.
[32] Wendler F. The LMTK-family of kinases: Emerging important players in cell physiology and disease pathogenesis [J]. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2018.



Comments
Leave a Comment