How Do You Get Exosomes?
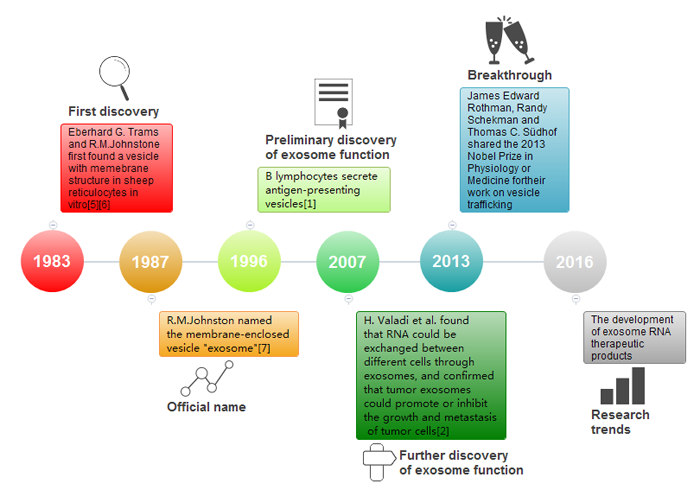
Exosomes were originally regarded as cellular metabolic waste. With the discovery of the antigen-presenting exosomes secreted by B lymphocytes involving anti-tumor responses in 1996 [1]and the exchange of genetic material between cells through RNA of the exosomes in 2007[2], people became aware of the importance of exosomes. These findings have also spurred
scientists to explore and study exosomes. And exosome research has been becoming a research hotspot since 2007[3][4].
Figure 1: The history of exosome research
Subsequently, more and more evidence shows that exosome analysis and detection can assist in early diagnosis, efficacy evaluation and prognosis analysis of diseases.
And scientists also have found exosomes can serve as vehicles to carry drugs, chemicals, or other molecules to the target tissue or organ for cancer treatment[8][9]
[10][11]. Specific exosomes are promising to be made into anti-cancer vaccines in the future. Furthermore, isolated exosomes are suitable for a wide range of
downstream analyses such as EM study, exosome label, exosome subpopulation, qRT-PCR profiling of exosomal miRNAs, and western blotting (WB).
Exosomes with high yield and purity are the basis of exosome research and the premise of guaranteeing the accuracy of research results. As exosomes widely exist in many body fluids such as
cerebrospinal fluid, saliva, amniotic fluid, ascites, blood, breast milk, and urine, so we must extract and purify exosomes from a broad spectrum of cellular debris and other interfering components.
Consequently, the isolation and purification of exosomes have become a major initiative in both basic research and clinical applications.
Five different exosomes isolation techniques have been developed capitalizing on the physicochemical and biochemical properties of exosomes. With the increasing demand for exosomes, various
exosome isolation and purification kits with good properties are emerging. Here mainly introduce five traditional exosome isolation methods and four exosome isolation kits in the following sections.
1. Five traditional exosome isolation methods:
Among the five exosome isolation methods, each method respectively exhibits a special exosomes' trait that aids their extraction such as density, shape, size, and
immunoaffinity (surface proteins).
1.1 Differential ultracentrifugation and Density gradient Ultracentrifugation
Ultracentrifugation is the most commonly used and reported exosome extraction method. It is the gold standard in exosome isolation. And it is popular in exosome research because of its easy
operation, no middle waiting periods of samples. However, its disadvantages are high requirements for centrifugal equipment and long run time. Ultracentrifugation is usually divided into two forms:
differential ultracentrifugation and density gradient ultracentrifugation.
Differential ultracentrifugation, also known as the pelleting method or simple ultracentrifugation method[12], gets exosome vesicles of similar size by using low-speed and high-speed
centrifugation alternately. It is based on the difference in the density and size of the exosomes and other components in the sample. It is easy to operate. But repeated centrifugation and large centrifugal
force may damage the structure of exosomes. The exosome yield is not high.
Density gradient ultracentrifugation is the variation of ultracentrifugation and contains two types: isopycnic ultracentrifugation and moving-zone ultracentrifugation. Isopycnic ultracentrifugation is
a speed-zone separation method by which different particles respectively sedimentate at a certain speed, forming discrete solute zone bands in a centrifuge tube with a density gradient medium under the
action of the ultracentrifugal force. The density gradient medium is pre-constructed in a centrifuge tube with progressively decreased density from bottom to top. Exosomes in the sample are enriched in the
appropriate density range (1.10-1.21g/ml)[13] and are finally harvested through brief ultracentrifugation of the density region of interest. The isolation technique is complicated, time-
consuming, and has strict requirements on centrifugation time. And it is largely affected by the narrow load zone. And collected exosomes are contaminated with similar-size vesicles[14] and
lost (large centrifugal force). But exosomes extracted in this way are relatively high in production.
Moving-zone ultracentrifugation is based on size and mass instead of density to separate exosomes. Different from isopycnic ultracentrifugation, the gradient density medium used in the moving-
zone ultracentrifugation has a lower density than that of any of the solutes.
1.2 Ultrafiltration method
Ultrafiltration method isolates exosomes by using the size characteristics of the exosomes (30 to 100 nm in diameter)[22][23]. It avoids the shortcomings of time consumption and
special equipment requirement in ultracentrifugation. This method is relatively simple & efficient and does not affect the biological activity of the
exosomes. But the isolated exosomes are not of high purity due to interference with other vesicles of similar size. Besides, exosomes may block the filter pores, resulting in a shorter membrane life and
lower separation efficiency[15]. Adhesion occurs between exosomes trapped on the membrane, leading to a decrease in yield[15].
1.3 Polypolymer precipitation method
Polypolymer precipitation method separates exosomes by precipitating exosomes due to the interaction between polyethylene glycol (PEG) and exosomes[14]. Because PEG can co-precipitate with
hydrophobic proteins or lipid molecules[16][17]. PEG was previously used to collect viruses from samples such as serum. The method is easy to operate and has a short cycle. But it also has
some problems such as low purity & recovery rate, high amount of heteroproteins (false positives), and uneven particle size[18][19][20].
1.4 Magnetic bead immunoassay
In the magnetic bead immunoassay (also known as antibody affinity purification), exosomes first bind to magnetic beads covered with marker protein antibodies of exosomes. These antibodies
specifically recognize exosomes' external biomarkers such as CD9 and CD63, etc. The complex exosomes-magnetic-antibody is absorbed and separated in the action of external
magnetic. The magnetic bead method has the advantages of high specificity, simple operation, and fewer impurities. But its production is relatively low, and exosome biological activity is easily affected by
pH and salt concentration. As a novel field, the best exosomal tags are still to be established[14]. Additionally, underestimations and false negatives may arise from tumor heterogeneity in
antigen expression and antigen modulations as the tumor progresses. Furthermore, the antigenic epitope may be blocked or masked[21].
1.5 Aqueous two phase system (ATPS) method
The aqueous two phase system (ATPS) is a liquid-liquid fractionation technique that works by taking advantage of the aqueous phase incompatibility of two polymer molecules. ATPS can separate particles
because different kinds of particles can be efficiently partitioned to different phases in a short time. Generally, an PEG/DEX aqueous two-phase system used to isolate exosomes[24]. Dissolve
polyethylene glycol and dextran in the sample and centrifugate it. The mixed solution is phase-separated after centrifugation. The top phase is PEG rich solution and the bottom phase is composed of
dextran rich solution. And exosomes are isolated into the bottom phase. In the TEM image, there is no morphology and size difference between the exosomes isolated using ATPS and those obtained using
ultracentrifugation, and all have intact lipid membranes[25]. And there is a minor shifting effect due to the dextran viscosity interfering in the analysis of RNA contents extracted from exosomes
isolated using ATPS and using ultra-centrifugation through a bio-analyzer[25]. ATPS is a simple, selective and low cost promising separation technology. However, insufficient understanding of
partition behavior is a major obstacle to the widespread application of ATPS at the industrial level for the purification of bio-molecules[26].
|
Exosome Isolation Methods
|
Operability
|
Operattion Time
|
Exosome Integrity
|
Exosome Purity
|
Exosome Yield
|
|
Differential ultracentrifugation
|
++++
|
Long
|
+
|
+
|
+++
|
|
Density gradient
ultracentrifugation
|
+++
|
Long
|
++
|
++
|
++++
|
|
Ultrafiltration
|
+++
|
Short
|
++++
|
++
|
++
|
|
Polypolymer precipitation
method
|
++++
|
Long
|
++++
|
++
|
+++
|
|
Magnetic bead
immunoassay
|
++
|
Short
|
++++
|
++
|
++
|
Table 1: Comparision of the exosome isolation methods
Of course, all these isolation methods are often used not alone but two or more together. In practical applications, the combination of several methods can give full play to the advantages of each
method while avoiding the disadvantages.
These conventional exosome separation methods are often limited by the resources of large instruments and equipment in the preparation of exosomes. And they have the defects of a complex
operation, time consumption, low yield & purity, and high cost. Therefore, traditional exosome separation methods are difficult to meet the demands of scientific research and clinical research on
exosomes. This provides a commanding impetus and growing demands for simple, fast, efficient, and affordable techniques to separate exosomes.
Based on conventional isolation methods, some researchers and biotech companies develop and design exosome isolation and purification kits. These kits perfectly compensate for the
disadvantages of traditional separation methods. Therefore, the kit method has gradually become the mainstream of exosome extraction methods, which can help most researchers to obtain high-quality
exosomes.
2. Four exosome isoaltion kits:
2.1 Exosome isolation kit containing a syringe filter
Exosomes isolated by ultrafiltration contain the most RNA in quantification by fluorescent staining. An exosome isolation kit is developed to isolate exosomes and then extract RNA from the
isolated exosomes from cell-free samples like urine, serum, cerebrospinal fluid. This mock ultrafiltration kit is a syringe filter tandemly configured with two membranes. When the sample passes through the
syringe filter, exosomes in the sample remain on the lower membrane, while larger extracellular vesicles retain on the upper membrane.
2.2 Exosome isolation kit based on precipitation method
The exosome isolation kit based on the precipitation method contains a hydrophilic polymer solution that precipitates exosomes and a sucrose density gradient reagent that enriches exosomes.
These kits enable fast and efficient enrichment of intact exosomes from various biological samples such as cell culture media, cerebrospinal fluid (CSF), ascitic fluid, amniotic fluid, milk, saliva, plasma &
serum, etc. A series of kit products of different article number correspond to different detection sample. It can exclude non-exosomes impurities such as high-abundance serum protein Albumin and IgG,
significantly improve the purity of the exosomes and effectively reduce the false positive rate that is beneficial to downstream proteomics.
2.3 Exosome isolation kit based on magnetic bead immunoassay
Tim4 protein can specifically recognize phosphatidylserine (PS) exposed on the outer of the exosome membrane. Some biological companies developed a novel exosome
purification kit with Tim4-immobilized magnetic beads, which help capture exosomes from almost all samples (body fluids, cell culture media samples, etc.) in the presence of calcium ions. Isolated
exosomes get purified by eluting with a chelating agent. However, exosomes collected by this kit may be mixed withTim4 and magnetic beads. There are also exosome isolation kits with magnetic beads
kits covered with antibodies that target exosome biomarker proteins such as CD63, CD9, or CD81 in the markets. And these kits are able to isolate exosomes from cell culture media samples.
2.4 Exosome isolation kit based on density gradient centrifugation
In the market, there is also exosome isolation and purification kit based on density gradient centrifugation. Exosomes, derived from the samples like cerebrospinal fluid (CSF), ascitic fluid, amniotic
fluid, milk, and saliva, are suspended in the middle fluffy layer of the density gradient medium reagent at the action of the centrifugation forces. Finally, exosomes are enriched and purified with the
molecular column. This kit avoids the contamination with artificial IgG and magnetic beads.
Exosome isolation kits achieve fast, convenient, and highly efficient
extraction of exosomes with high purity and yield from the various sample solutions. Isolated exosomes using kits methods remain intact, maintaining the bioactivity of exosomes. Intact, pure, and high-
producing exosomes provide a more stable and reliable guarantee for exosome research.
References:
[1] G. Raposo, H.W. Nijman, W. Stoorvogel, R. Liejendekker, C.V. Harding, C.J. Melief, H.J. Geuze. B lymphocytes secrete antigen-presenting vesicles [J]. J. Exp. Med. 1996,
183:1161–1172.
[2]H. Valadi, A. Bossios, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells [J]. Nat. Cell Biol.
2007, 9:654–659.
[3]Zong SF, Wang L, et al. Facile detection of tumor-derived exosomes using magnetic nanobeads and SERS nanoprobes [J]. Anal Methods. 2016, 8:5001–08.
[4] Harding CV, Heuser JE, et al. Exosomes: looking back three decades and into the future [J]. J. Cell Biol. 2013, 200:367–371.
[5] B.T. Pan, R.M. Johnstone. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor [J]. Cell. 1983,
33:967–978.
[6] Pan BT, Teng K, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes [J]. J. Cell
Biol.1985;101:942–948.
[7] Johnstone RM, Bianchini A, et al. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane
functions [J]. Blood. 1989, 74:1844–1851.
[8] Safdar A, Saleem A, et al. The potential of endurance exercise-derived exosomes to treat metabolic diseases [J]. Nat Rev Endocrinol. 2016, 12:504–17.
[9]Laulagnier K, Motta C, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization [J]. Biochem. J. 2004, 380:161–171.
[10] Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol. Lett. 2006, 107:102–108.
[11] Li J, Yang X, et al. Exosome-derived microRNAs contribute to prostate cancer chemoresistance [J]. Int J Oncol. 2016, 49:838–46.
[12] Yamashita T, Takahashi Y, et al. Effect of exosome isolation methods on physicochemical properties of exosomes and clearance of exosomes from the blood
circulation [J]. Eur. J. Pharm. 2016, 98:1–8.
[13] Miranda KC, Bond DT, et al. Massively parallel sequencing of human urinary exosome/microvesicle RNA reveals a predominance of non-coding RNA
[J]. PloS ONE. 2014, 9:e96094.
[14] Zeringer E, Barta T, et al. Strategies for isolation of exosomes [J]. Cold Spring Harb. Protoc; 2015, pp. 319–323.
[15] Liga A, Vliegenthart AD, et al. Exosome isolation: a microfluidic road-map [J]. Lab Chip. 2015, 15:2388–2394.
[16] Kim D, Nishida H, et al. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after
TBI [J]. Proc. Natl. Acad. Sci. USA. 2016, 113:170–175.
[17] Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery [J]. J. Control Release. 2015;219:396–405.
[18]Zarovni N, Corrado A, et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches
[J]. Methods. 2015, 87:46–58.
[19] Kalra H, Adda CG, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal
human blood plasma [J]. Proteomics. 2013, 13:3354–3364.
[20]Gyorgy B, Modos K, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical
parameters [J]. Blood. 2011, 117:e39–48.
[21] Quintana JF, Makepeace BL, et al. Extracellular Onchocerca-derived small RNAs in host nodules and blood [J]. Parasit. Vectors. 2015, 8:1.
[22] Lobb RJ, Becker M, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma [J]. J. Extracell. Vesicles. 2015,
4:27031.
[23] Valcz G, Galamb O, et al. Exosomes in colorectal carcinoma formation: ALIX under the magnifying glass [J]. Mod Pathol. 2016, 29:928-38.
[24] Shin, H. et al. High-yield isolation of extracellular vesicles using aqueous two-phase system [J]. Sci. Rep. 2015, 5, 13103.
[25] Jongmin Kim,Hyunwoo Shin, et al. Isolation of High-Purity Extracellular Vesicles by Extracting Proteins Using Aqueous Two-Phase System [J]. PLoS ONE 2015, 10(6): e0129760.
[26] Perez B, Malpiedi LP, et al. Experimental determination and thermodynamic modeling of phase equilibrium and protein partitioning in aqueous two-phase systems containing biodegradable salts [J]. J
Chem Thermodyn. 2013; 56:136–43.
Cite this article
CUSABIO team. How Do You Get Exosomes?. https://www.cusabio.com/c-20965.html




Comments
Leave a Comment