Recently, CD38 causes fresh concerns in the field of anti-aging because two articles published in Nature Metabolis point that CD38 attributes to the NAD+ consumption [1-2]. NAD support is called by some as the "immortality molecule", but NAD+ levels usually decline during aging. The articles indicate that CD38 might function as a dormant switch for the human age. Despite the possibility not yet clinically identified, the roles of CD38 in cancer immunotherapy has been widely clinically proven. The number of confirmed cases provide important clinical evidence for CD38-targeted immunotherapeutic approaches in cancer. Thus, what is this amazing CD38 and what important functions does it perform in tumor therapies? What's the specified role of CD38 in aging? With questions, let's get real about CD38.
1. CD38 Structure and Enzymatic Functions
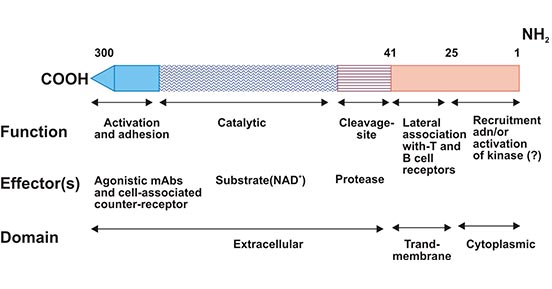
CD38 is a type II transmembrane glycoprotein with a molecular weight of 46 kDa. CD38 was first identified in the early 1980s by E. L. Reinherz and S. F. Schlossman. CD38 has a short amino-terminal cytoplasmic tail, a single membrane-spanning region, and a long extracel-lular carboxy-terminal domain (Figure 1) [3]. CD38 is a bifunctional ectoenzyme that uses nicotinamide adenine dinucleotide (NAD) as a substrate to generate second messengers like cyclic ADP ribose (cADPR) [4, 5], which contribute to the Ca2+ mobilization. The Ca2+ mobilization further activates the signaling pathways that control various biological processes such as insulin secretion and pancreatic β-cell proliferation [6].
Meanwhile, CD38 plays important roles in maintaining the dynamic balance of NAD and its metabolites levels. It has also been found that CD38, together with CD39, CD73, CD203a, etc., induces a suppressive immune microenvironment by degrading adenosine triphosphate/ATP, NAD+, cADPR, and adenosine monophosphate/AMP, which leads to the production of adenosine/ADO [7].
Figure 1. Schematic representation of CD38 structure
*This figure is derived from the publication on FASEB Journal [3]
CD38 is expressed in a variety of human tissues, including lymphocytes, natural killer cells, T cells, B-cell monocytes/macrophages pancreas, brain, spleen, and liver cells, etc [8, 9]. Interestingly, the levels of CD38 expression have been correlated with a variety of diseases, including acquired immunodeficiency syndrome/AIDS, autoimmune diseases (e.g., systemic lupus erythematosus, SLE), type 2 diabetes, osteoporosis, and cancer [10-12].
2. CD38 in Non-Solid Tumors
In non-solid tumors, CD38 is considered as a predictor of B-cell chronic lymphocytic leukemia (B-CLL) given its overexpression. B-CLL patients who suggested high expression of CD38 in the B lymphocytes of peripheral blood is strongly associated with poor prognosis [13]. Others, include Waldenström's macroglobulinemia [14], systemic light-chain (AL) amyloidosis [15], mantle cell lymphoma/MCL [16], acute myeloid leukemia/AML [17], acute lymphoblastic leukemia/ALL [18], and NK cell leukemia [19]. Among these non-solid tumors, high expression of CD38 is identified.
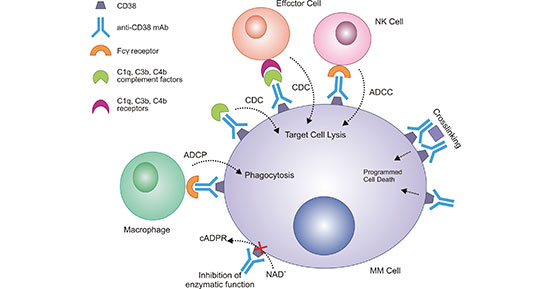
Figure 2. Mechanism of CD38 monoclonal antibody in multiple myeloma
*This figure is derived from the publication on Frontiers in immunology [20]
Notably, CD38 on multiple myeloma (MM) cells, its expression is very high, which suppresses immunity through its enzymatic activity [20]. In contrast, in normal lymphocytes and myeloid cells, CD38 expression is low. As described in Figure 2, monoclonal antibodies targeting the CD38 in MM cells can trigger a variety of different mechanisms to promote apoptosis and modulate the immune response [20].
3. CD38 in Solid Tumors
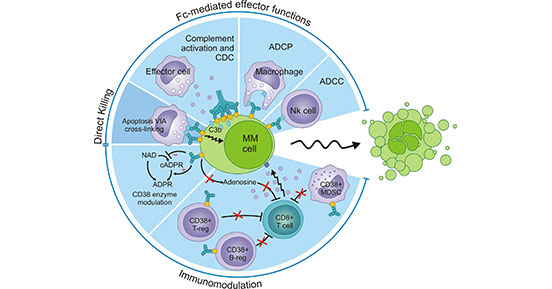
In some solid tumors, high levels of CD38 expression is associated with the increased adenosine [21]. It is well-recognized that adenosine has a marked dampening effect on the immune response. As represented in Figure 3, CD38 overexpression contributes to the production of adenosine in the tumor microenvironment. Then adenosine recruits immunosuppressive cells such as Treg cells, myeloid-derived suppressor cell/MDSC cells, cancer-associated fibroblast/CAF cells, etc., which results in disorders of the immune system. Further, adenosine binds to its receptor A2AR on the surface of immune cells to activate inhibitory signaling pathways [22]. All in all, CD38 might suppress immune response such as CD8+T cells via adenosine receptor signaling.
Figure 3. CD38 signaling pathway in the solid tumors
*This figure is derived from the publication on Cells [22]
Although the specified mechanisms of CD38 in solid tumors remain to be further explored, several studies have shed some light on the roles of CD38 in melanoma, glioma, esophageal, cervical, and lung cancers. In these diseases, CD38 appears to function as a tumor-promoting factor [23].
4. CD38 in the Age-Related NAD Decline
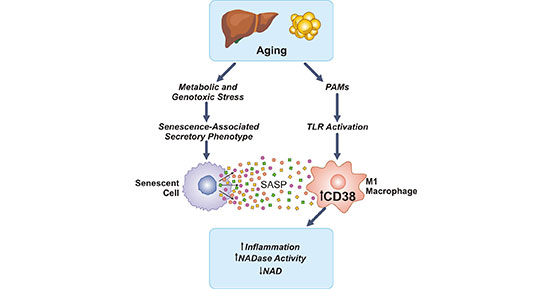
The aforementioned studies published in Nature Metabolism found that mice lacking CD38 were protected from age-related NAD decline and had enhanced metabolic health [2]. The findings raised the possibility that with an increasing burden of senescent cells, which is also implicated in the aging process, cause the degradation of NAD via the activation of CD38 (Figure 4) [1].
Figure 4. CD38 in the age-related NAD decline
*This figure is derived from the publication on Nature Metabolism [21]
NAD+ is a cellular coenzyme that's found in every cell of our bodies. Maintenance of NAD+ levels is important for anti-aging. Preliminary data suggest that blocking the activity of CD38 in aged animals can restore NAD+ levels in specific tissues [2]. Therefore, targeting CD38 may provide scientists with another way to address the decline in NAD+ against aging.
5. CD38-Targeting Antibody for Clinical Uses
In recent years, several novel immunotherapies targeting CD38 have been validated in preclinical models and clinical trials. In 2015, Daratumumab (Darzalex), a fully human anti-CD38 antibody developed by Johnson & Johnson, was approved for the treatment of patients with relapsed, drug-resistant MM. Daratumumab became the first CD38 antibody on the market.
On March 2, 2020, Sanofi received FDA approval for its oncology drug Isatuximab, the second CD38 antibody drug after Daratumumab, also for the treatment of MM treatment. Currently, both Daratumumab and Isatuximab show significant efficacy in MM therapy.
It is worth mentioning that several pharmaceutical companies, including China's Sumgen and YZY Biopharma, have also started the development of antibodies targeting CD38. Recently, CASI Pharmaceutical submitted an Investigational New Drug application (IND) for CD38 antibody. More pharmaceutical companies are in a highly competitive race, as shown in the table below.
|
Name
|
Research Phase
|
Company
|
Indications
|
Last Updated Date
|
|
Isatuximab
|
Listing Approval
|
Immunogen Inc;
|
Multiple myeloma;
Multiple myeloma of the heart;
Acute lymphocytic leukemia; acute myeloid leukemia;
Acute myeloid leukemia;
|
2020-11-06
|
|
Daratumumab/Hyaluronidase-fihj
|
Listing Approval
|
Janssen;
|
Multiple Myeloma;
|
2020-08-28
|
|
Daratumumab (Daretuzumab)
|
Listing Approval
|
Janssen;
|
Multiple myeloma;
Amyloidosis;
amyloidosis; cathartic multiple myeloma;
Acute lymphocytic leukemia; Extra-nodal NK-T cell lymphoma
|
2020-08-28
|
|
MOR-202
|
Clinical Phase III
|
Morphosys Ag;
|
Multiple myeloma;
Membranous glomerulonephritis;
|
2020-08-28
|
|
TAK-573
|
Clinical Phase II
|
Takeda Pharmaceutical Co Ltd;
|
Multiple myeloma;
Non-small cell lung cancer;
Melanoma;
Prostate cancer;
Triple-negative breast tumors;
Head and neck cancer;
Ductal carcinoma of pancreas;
|
2020-08-28
|
|
TAK-079
|
Clinical Phase II
|
Takeda Pharmaceutical Industry Co;
|
Idiopathic thrombocytopenic purpura;
Severe myasthenia gravis;
Multiple myeloma;
Relapses;
Systemic lupus erythematosus;
Autoimmune diseases;
|
2020-08-28
|
|
FT-500
|
Clinical Phase I
|
Fate therapeutics, University of Minnesota
|
Solid Tumor
|
2020-09-28
|
|
AMG-424
|
Clinical Phase I
|
Amgen Inc;
Xencor;
|
Multiple myeloma;
Recurrence;
|
2020-09-07
|
|
STI-6129
|
Clinical Phase I
|
|
Amyloid light chain amyloidosis
|
2020-09-27
|
|
Anti CD38 chimeric antigen receptor T-cell therapy (Sorrento Therapeutics)
|
Clinical Phase I
|
Celularity;
Sorrento Therapeutics;
|
Multiple Myeloma;
|
2020-08-28
|
|
GBR-1342
|
Clinical Phase I
|
Glenmark Pharmaceuticals;
|
Multiple Myeloma;
|
2020-08-28
|
|
Anti-BCMA anti-CD38 bispecific chimeric antigen receptor T cell therapy
|
Clinical Phase I
|
Shengyan Pharmaceutical Technology;
Wuhan Xiehe Hospital;
|
Multiple Myeloma;
|
2020-08-28
|
|
Recombinant human anti-CD38 momoclonal antibody
|
Clinical Applications
|
Hangzhou Shangjian Biotechnology Co;
|
Blood Tumors;
|
2020-08-28
|
|
Y-150
|
Clinical Applications
|
Wuhan Youzhiyou Biopharmaceutical Co;
|
Multiple Myeloma;
|
2020-08-28
|
|
CID-103
|
Clinical Applications
|
Tusk Therapeutics; CASI pharmaceuticals
|
Multiple Myeloma;
Hematologic tumors;
|
2020-08-28
|
*This data derives from pharmacodia.
6. CD38 Potential Perspectives
Apparently, antibody drugs have a wide application in cancer and autoimmune diseases. The development history of CD38 antibody drugs can be traced back to the 1990s, but currently only two CD38 monoclonal antibodies have been approved for marketing. According to Johnson & Johnson's latest earnings report, global sales of Darzalex reached $2.9 billion in 2019. It is said that global sales of Darzalex are expected to reach $6 billion in 2024, analysis on Pharma.
The expression of CD38 in immune cells suggests important roles in immune response, which provides a powerful weapon for the MM treatment. MM, a disease that affects more than 130,000 patients worldwide each year, antibodies targeting CD38 will not only fight tumors but also have potential value in the treatment of aging-related conditions such as fibrosis and metabolic disorders. Therefore, the development of CD38-targeted drugs is becoming more attractive for big pharmaceutical companies.
References
[1] Covarrubias AJ, Kale A, Perrone R, et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages [J]. Nature Metabolism. 2020, 2(11):1265-83.
[2] Chini CC, Peclat TR, Warner GM, et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels [J]. Nature Metabolism. 2020, 2(11):1284-304.
[3] Mehta, K., Shahid, U., & Malavasi, F. Human CD38, a cell-surface protein with multiple functions [J]. FASEB journal. 1996, 10(12): 1408-1417.
[4] Durnin L, Mutafova‐Yambolieva VN. Cyclic ADP-ribose requires CD38 to regulate the release of ATP in visceral smooth muscle [J]. FEBS. 2011, 278(17): 3095-108.
[5] Chini EN. CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions [J]. Current pharmaceutical design. 2009, 15(1):57-63.
[6] Navazio L, Mariani P, Sanders D. Mobilization of Ca2+ by cyclic ADP-ribose from the endoplasmic reticulum of cauliflower florets [J]. Plant Physiology. 2001, 1; 125(4):2129-38.
[7] Morandi F, Airoldi I, Marimpietri D, Bracci C, Faini AC, Gramignoli R. CD38, a Receptor with Multifunctional Activities: From Modulatory Functions on Regulatory Cell Subsets and Extracellular Vesicles, to a Target for Therapeutic Strategies [J]. Cells. 2019, 8(12):1527.
[8] Glaría E, Valledor AF. Roles of CD38 in the Immune Response to Infection [J]. Cells. 2020, 9(1):228.
[9] Guerreiro S, Privat AL, Bressac L, Toulorge D. CD38 in Neurodegeneration and Neuroinflammation [J]. Cells. 2020, 9(2):471.
[10] Cole S, Walsh A, Yin X, Wechalekar MD, et al. Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus [J]. Arthritis research & therapy. 2018, 20(1):1-4.
[11] Savarino A, Bottarel F, Malavasi F, Dianzani U. Role of CD38 in HIV-1 infection: an epiphenomenon of T-cell activation or an active player in virus/host interactions? [J]. Aids. 2000, 14(9):1079-89.
[12] van de Donk NW, Janmaat ML, et al. Monoclonal antibodies targeting CD 38 in hematological malignancies and beyond [J]. Immunological reviews. 2016, 270(1):95-112.
[13] Dürig J, Naschar M, Schmücker U, et al. CD38 expression is an important prognostic marker in chronic lymphocytic leukaemia [J]. Leukemia. 2002, 16(1):30-5.
[14] Paulus A, Manna A, Akhtar S, et al. Targeting CD38 with daratumumab is lethal to Waldenström macroglobulinaemia cells [J]. British Journal of Haematology. 2018, 183(2):196-211.
[15] Roccatello D, Fenoglio R, Sciascia S, et al. CD38 and Anti-CD38 Monoclonal Antibodies in AL Amyloidosis: Targeting Plasma Cells and beyond [J]. International Journal of Molecular Sciences. 2020, 21(11):4129.
[16] Strati P, Shanafelt TD. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification [J]. Blood. 2015, 23; 126 (4):454-62.
[17] Naik J, Themeli M, de Jong-Korlaar R, et al. CD38 as a therapeutic target for adult acute myeloid leukemia and T-cell acute lymphoblastic leukemia [J]. Haematologica. 2019, 104(3):e100.
[18] George AA, Franklin J, Kerkof K, et al. Detection of leukemic cells in the CD34+ CD38− bone marrow progenitor population in children with acute lymphoblastic leukemia [J]. Blood, the Journal of the American Society of Hematology. 2001, 97(12):3925-30.
[19] Mallone R, Funaro A, Zubiaur M, et al. Signaling through CD38 induces NK cell activation [J]. International immunology. 2001, 13(4):397-409.
[20] Morandi F, Horenstein AL, Costa F, et al. CD38: a target for immunotherapeutic approaches in multiple myeloma [J]. Frontiers in immunology. 2018, 9:2722.
[21] Fang C, Li T, Li Y, et al. CD38 produces nicotinic acid adenosine dinucleotide phosphate in the lysosome [J]. Journal of Biological Chemistry. 2018, 293(21):8151-60.
[22] Janmaat ML, van de Donk NW, van Bueren JL, et al. Discovery, Development, and Mechanisms of Action of the Human CD38 Antibody Daratumumab. Successful Drug Discovery. 2018, 3:153-95.
[23] Konen JM, Fradette JJ, Gibbons DL. The Good, the Bad and the Unknown of CD38 in the Metabolic Microenvironment and Immune Cell Functionality of Solid Tumors [J]. Cells. 2020, 9(1):52.
CUSABIO team. The Multifunctional Enzyme CD38: A Target for Immunotherapeutic Approaches in Cancer. https://www.cusabio.com/c-20997.html




Comments
Leave a Comment