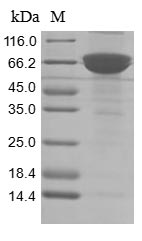
Recombinant human ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (CD38) production in mammalian cells involves co-cloning the gene encoding the extracellular domain of the human CD38 protein (43-300aa) into an expression vector with a C-terminal hFc-tag gene, followed by transformation into mammalian cells. The cells are cultured under conditions that induce protein expression. After sufficient growth, the cells are lysed to release the recombinant protein. Purification is achieved using affinity chromatography technique. The purity of the CD38 protein is confirmed using SDS-PAGE, reaching over 85%.
Human CD38 protein is a transmembrane glycoprotein that stimulates B-lymphocyte activation and acts as an enzyme that catalyzes the synthesis of cyclic ADP-ribose from NAD+ [1]. It is a part of the ADP ribosyl cyclase/CD38 gene family and is evolutionarily conserved, indicating its importance in human physiology [2]. CD38 functions as an ectoenzyme and a receptor, degrading NAD and modulating cellular NAD homeostasis [3]. Furthermore, CD38 is involved in oxytocin secretion, social behavior, and calcium regulation [4][5]. It is expressed in inflammatory conditions and is robustly induced in human macrophages under inflammatory stimuli [6]. CD38 is also implicated in diseases like cancer, where it is methylated in prostate cancer and regulates extracellular NAD+ [7].
References:
[1] M. Dong, Y. Qi, S. Sun, X. Pu, Z. Yang, L. Zhanget al., Design, synthesis and biological characterization of novel inhibitors of cd38, Organic & Biomolecular Chemistry, vol. 9, no. 9, p. 3246, 2011. https://doi.org/10.1039/c0ob00768d
[2] F. Malavasi, S. Deaglio, A. Funaro, E. Ferrero, A. Horenstein, E. Ortolanet al., Evolution and function of the adp ribosyl cyclase/cd38 gene family in physiology and pathology, Physiological Reviews, vol. 88, no. 3, p. 841-886, 2008. https://doi.org/10.1152/physrev.00035.2007
[3] E. Chini, C. Chini, J. Netto, G. Oliveira, & W. Schooten, The pharmacology of cd38/nadase: an emerging target in cancer and diseases of aging, Trends in Pharmacological Sciences, vol. 39, no. 4, p. 424-436, 2018. https://doi.org/10.1016/j.tips.2018.02.001
[4] H. Higashida, S. Yokoyama, M. Kikuchi, & T. Munesue, Cd38 and its role in oxytocin secretion and social behavior, Hormones and Behavior, vol. 61, no. 3, p. 351-358, 2012. https://doi.org/10.1016/j.yhbeh.2011.12.011
[5] V. Sathish, M. Thompson, S. Sinha, G. Sieck, Y. Prakash, & C. Pabelick, Inflammation, caveolae and cd38-mediated calcium regulation in human airway smooth muscle, Biochimica Et Biophysica Acta (Bba) - Molecular Cell Research, vol. 1843, no. 2, p. 346-351, 2014. https://doi.org/10.1016/j.bbamcr.2013.11.011
[6] S. Amici, M. Young, J. Narvaez-Miranda, K. Jablonski, J. Arcos, L. Rosaset al., Cd38 is robustly induced in human macrophages and monocytes in inflammatory conditions, Frontiers in Immunology, vol. 9, 2018. https://doi.org/10.3389/fimmu.2018.01593
[7] J. Mottahedeh, M. Haffner, T. Grogan, T. Hashimoto, P. Crowell, H. Beltranet al., Cd38 is methylated in prostate cancer and regulates extracellular nad+, Cancer & Metabolism, vol. 6, no. 1, 2018. https://doi.org/10.1186/s40170-018-0186-3