[1] Gay, Nathan D., et al. "Durable response to afatinib in lung adenocarcinoma harboring NRG1 gene fusions." Journal of Thoracic Oncology 12.8 (2017): e107-e110.
[2] Muscarella, Lucia Anna, et al. "ALK and NRG1 fusions coexist in a patient with signet ring cell lung adenocarcinoma." Journal of Thoracic Oncology 12.10 (2017): e161-e163.
[3] Cheema, Parneet K., Mark Doherty, and Ming-Sound Tsao. "A case of invasive mucinous pulmonary adenocarcinoma with a CD74-NRG1 fusion protein targeted with afatinib." Journal of Thoracic Oncology 12.12 (2017): e200-e202.
[4] Rimer, Mendell. "Neuregulins at the neuromuscular synapse: past, present, and future." Journal of neuroscience research 85.9 (2007): 1827-1833.
[5] Dammann, Christiane EL, Heber C. Nielsen, and Kermit L. Carraway III. "Role of neuregulin-1β in the developing lung." American journal of respiratory and critical care medicine 167.12 (2003): 1711-1716.
[6] Zhao, Weijiang. "Neuregulin-1 (Nrg1): An emerging regulator of prolactin (PRL) secretion." Prolactin (2013): 83.
[7] Wansbury, Olivia, et al. "Dynamic expression of Erbb pathway members during early mammary gland morphogenesis." Journal of investigative dermatology 128.4 (2008): 1009-1021.
[8] Sithanandam, G., and L. M. Anderson. "The ERBB3 receptor in cancer and cancer gene therapy." Cancer gene therapy 15.7 (2008): 413-448.
[9] Fock, Valerie, et al. "Neuregulin-1-mediated ErbB2-ErbB3 signalling protects human trophoblasts against apoptosis to preserve differentiation." Journal of cell science 128.23 (2015): 4306-4316.
[10] Wadugu, Brian, and Bernhard Kühn. "The role of neuregulin/ErbB2/ErbB4 signaling in the heart with special focus on effects on cardiomyocyte proliferation." American Journal of Physiology-Heart and Circulatory Physiology 302.11 (2012): H2139-H2147.
[11] Dimou, Anastasios, and D. Ross Camidge. "Detection of NRG1 fusions in solid tumors: Rare gold?" Clinical Cancer Research 25.16 (2019): 4865-4867.
[12] Liu, Dawei, and Xinhong Luan. "The Research Progress of Neuregulin 1 in the Reproductive Development." (2015).
[13] Newbern, Jason, and Carmen Birchmeier. "Nrg1/ErbB signaling networks in Schwann cell development and myelination." Seminars in cell & developmental biology. Vol. 21. No. 9. Academic Press, 2010.
[14] Xu, Junping, et al. "Neuregulin-1 protects mouse cerebellum against oxidative stress and neuroinflammation." Brain Research 1670 (2017): 32-43.
[15] Shamir, Alon, et al. "The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders." Journal of Neuroscience 32.9 (2012): 2988-2997.
[16] Munafo, M. R., et al. "Association of the NRG1 gene and schizophrenia: a meta-analysis." Molecular psychiatry 11.6 (2006): 539-546.
[17] Pascual-Gil, S., et al. "NRG1 PLGA MP locally induce macrophage polarisation toward a regenerative phenotype in the heart after acute myocardial infarction." Journal of drug targeting 27.5-6 (2019): 573-581.
[18] Jones, M. R., et al. "Successful targeting of the NRG1 pathway indicates novel treatment strategy for metastatic cancer." Annals of Oncology 28.12 (2017): 3092-3097.
[19] Weinstein, Edward J., Stefan Grimm, and Philip Leder. "The oncogene heregulin induces apoptosis in breast epithelial cells and tumors." Oncogene 17.16 (1998): 2107-2113.
[20] Chua, Y. L., et al. "The NRG1 gene is frequently silenced by methylation in breast cancers and is a strong candidate for the 8p tumour suppressor gene." Oncogene 28.46 (2009): 4041-4052.
[21] Gladstone, E., et al. "MA21. 01 Generation and Characterization of Novel Preclinical Disease Models of NSCLC with NRG1 Rearrangements to Improve Therapy." Journal of Thoracic Oncology 14.10 (2019): S334.
[22] Stahler, Arndt, et al. "Prevalence and influence on outcome of HER2/neu, HER3 and NRG1 expression in patients with metastatic colorectal cancer." Anti-cancer drugs 28.7 (2017): 717-722.
[23] SJonna, Sushma, et al. "Detection of NRG1 gene fusions in solid tumors." Clinical Cancer Research 25.16 (2019): 4966-4972.

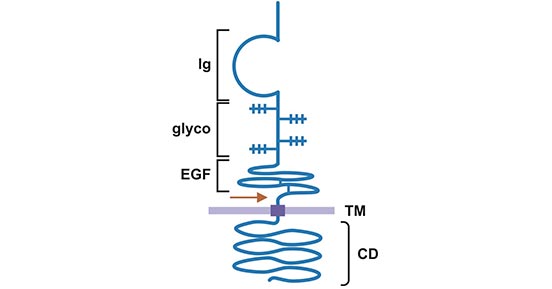
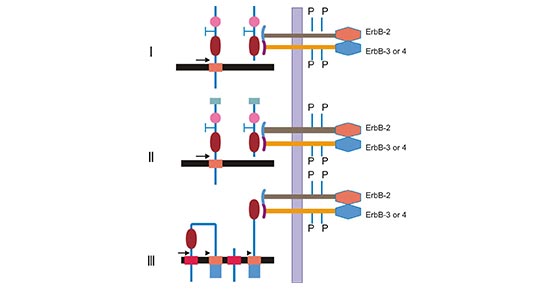
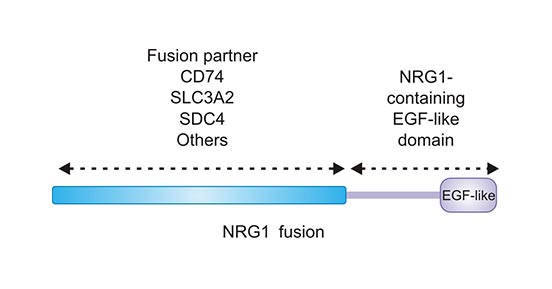
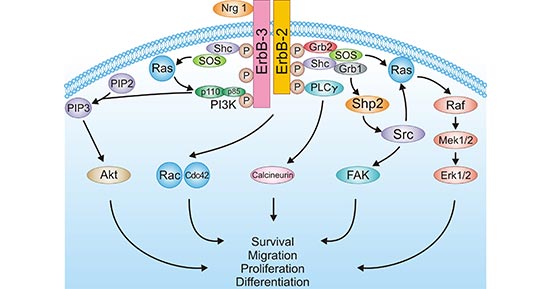
-SDS.jpg)
-AC1.jpg)



Comments
Leave a Comment