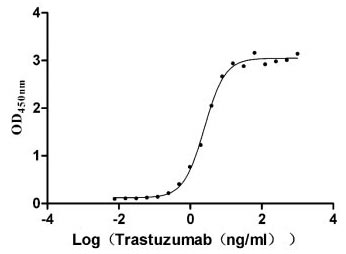
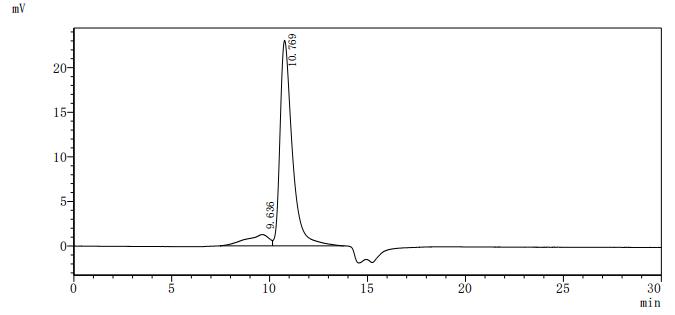
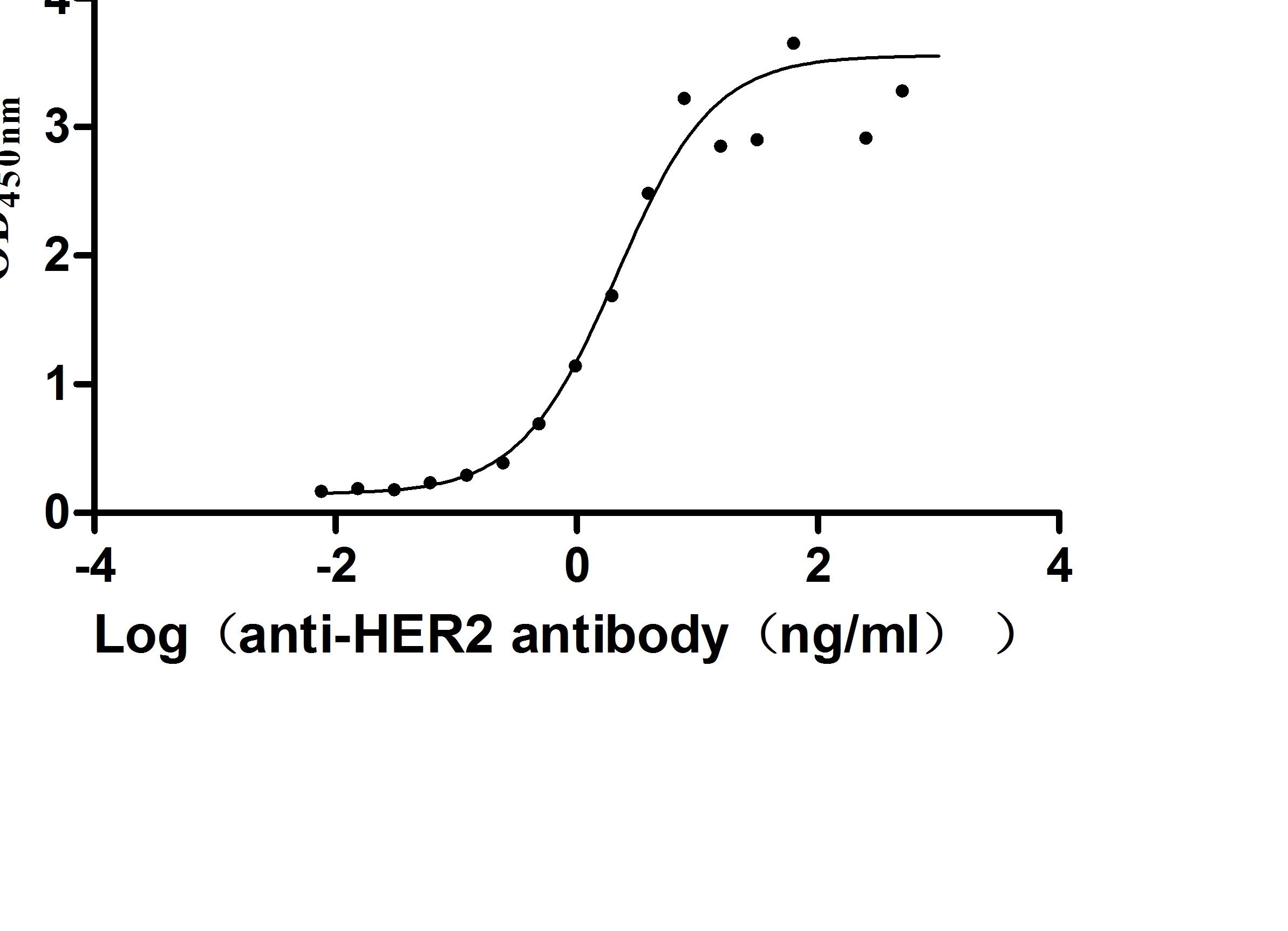
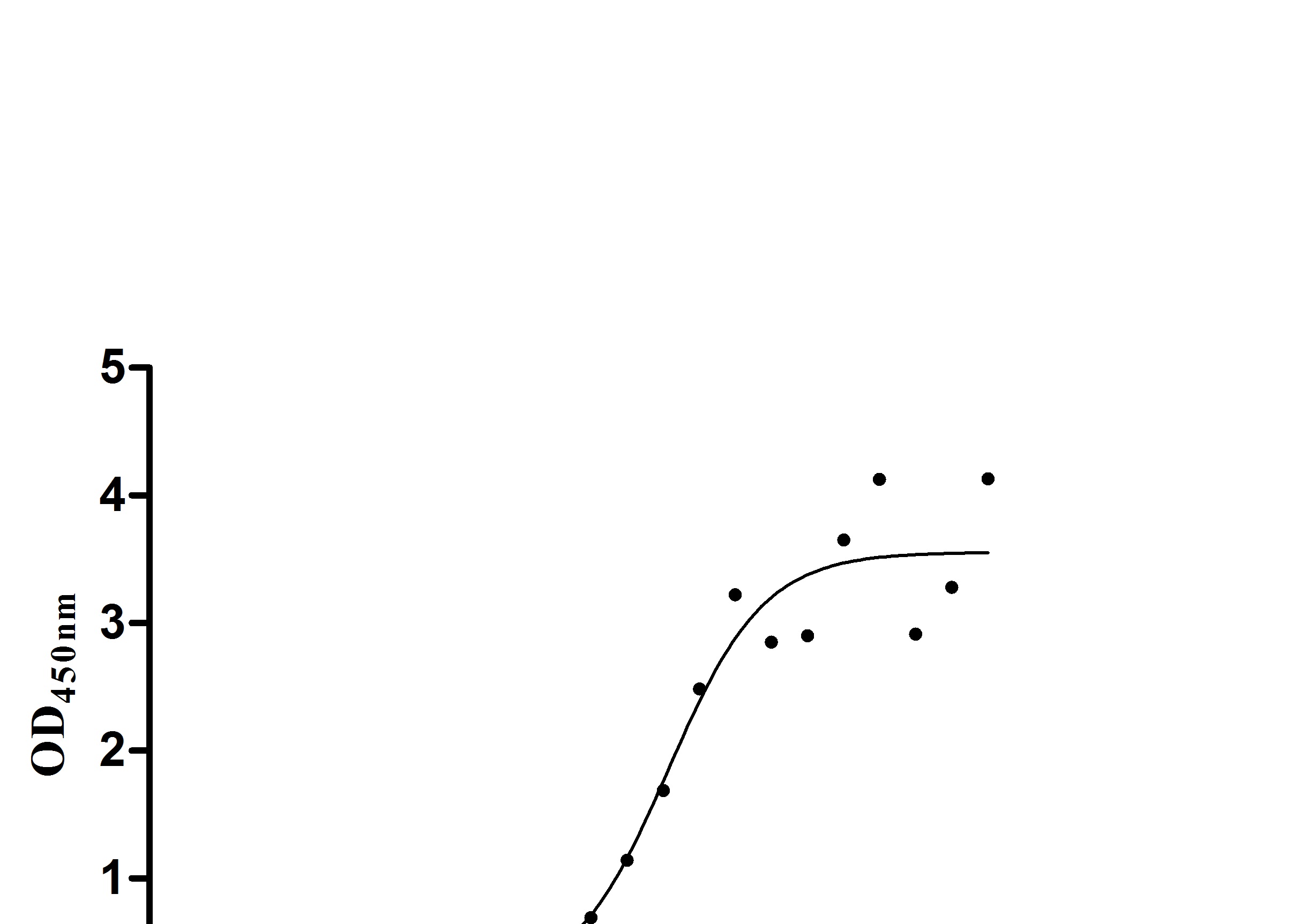
The recombinant human ERBB2 protein is generated in mammalian cells through the expression of the plasmid containing the target gene that encodes the 23-652aa of the human ERBB2. This protein is fused with 6xHis-tag at the C-terminus. The protein achieves a purity of over 95%, verified by SDS-PAGE, and contains endotoxin less than 1.0 EU/μg, measured via the LAL method. It has been validated to be biologically active. ELISA confirms its specific binding to the Trastuzumab, with an EC50 ranging from 2.179 to 2.825 ng/mL.
Human ERBB2, also known as HER2/neu, a member of the EGFR family, is a transmembrane tyrosine kinase involved in cell signaling pathways that regulate cell proliferation, survival, and differentiation. ERBB2 is particularly notable for its role in various cancers, most prominently breast cancer, where it is overexpressed in approximately 15-30% of cases, leading to aggressive tumor behavior and poor prognosis [1][2][3].
The ERBB2 protein lacks a known ligand but is essential for forming heterodimers with other EGFR family members, particularly with HER3. This dimerization enhances signaling through pathways such as the PI3K/Akt and MAPK pathways, which are critical for promoting cell survival and proliferation [1][4][5][6]. The aberrant activation of these signaling pathways due to ERBB2 overexpression is a significant contributor to oncogenesis and tumor progression [7][8][9].
In addition to its role in cancer, ERBB2 has been implicated in various physiological processes, including cardiac function. Studies have shown that ERBB2 is involved in cardiomyocyte survival and hypertrophy [10][11]. Therapeutically, ERBB2 has become a target for monoclonal antibody treatments, most notably trastuzumab (Herceptin), which has been shown to improve outcomes in patients with ERBB2-positive breast cancer by inhibiting receptor signaling and promoting antibody-dependent cellular cytotoxicity [1][4][12].
References:
[1] M. Karass, R. Bareja, E. Shelkey, P. Vlachostergios, B. Robinson, F. Khani, et al. Oncogenic addiction to erbb2 signaling predicts response to trastuzumab in urothelial cancer, Journal of the National Comprehensive Cancer Network, vol. 17, no. 3, p. 194-200, 2019. https://doi.org/10.6004/jnccn.2018.7264
[2] E. Zhang, M. Cristofanilli, F. Robertson, J. Reuben, Z. Mu, R. Beavis, et al. Genome wide proteomics of erbb2 and egfr and other oncogenic pathways in inflammatory breast cancer, Journal of Proteome Research, vol. 12, no. 6, p. 2805-2817, 2013. https://doi.org/10.1021/pr4001527
[3] Y. Li, Y. Pan, Y. Wei, X. Cheng, B. Zhou, M. Tan, et al. Upregulation of cxcr4 is essential for her2-mediated tumor metastasis, Cancer Cell, vol. 6, no. 5, p. 459-469, 2004. https://doi.org/10.1016/j.ccr.2004.09.027
[4] J. Liu, T. Wang, C. Willson, K. Janardhan, S. Wu, J. Li, et al. Erbb2 regulates med24 during cancer progression in mice with pten and smad4 deletion in the pulmonary epithelium, Cells, vol. 8, no. 6, p. 615, 2019. https://doi.org/10.3390/cells8060615
[5] L. Arias-Romero, O. Villamar-Cruz, A. Pacheco, R. Kosoff, M. Huang, S. Muthuswamy, et al. A rac–pak signaling pathway is essential for erbb2-mediated transformation of human breast epithelial cancer cells, Oncogene, vol. 29, no. 43, p. 5839-5849, 2010. https://doi.org/10.1038/onc.2010.318
[6] X. Liu, Y. Zhang, W. Ren, & G. Rao. Erbb2 gene silencing and its effect on pten in sacc-83 salivary adenoid cystic carcinoma cells, Oncology Reports, vol. 24, no. 5, 2010. https://doi.org/10.3892/or_00000985
[7] E. Wolfson, M. Goldenberg, S. Solomon, A. Frishberg, & R. Pinkas‐Kramarski. Nucleolin-binding by erbb2 enhances tumorigenicity of erbb2-positive breast cancer, Oncotarget, vol. 7, no. 40, p. 65320-65334, 2016. https://doi.org/10.18632/oncotarget.11323
[8] Y. Qi, T. Su, Z. Xia, Y. Jiang, W. Yuan, W. Wang, et al. Gene expression profiles of phaeochromocytomas with erbb2 overexpression reveal a new molecular mechanism tumourigenicity, Clinical Endocrinology, vol. 77, no. 3, p. 399-406, 2012. https://doi.org/10.1111/j.1365-2265.2012.04388.x
[9] S. Karakashev and M. Reginato. Hypoxia/hif1α induces lapatinib resistance in erbb2-positive breast cancer cells via regulation of dusp2, Oncotarget, vol. 6, no. 4, p. 1967-1980, 2015. https://doi.org/10.18632/oncotarget.2806
[10] P. Sysa‐Shah, Y. Xu, X. Guo, F. Belmonte, B. Kang, D. Bedja, et al. Cardiac-specific over-expression of epidermal growth factor receptor 2 (erbb2) induces pro-survival pathways and hypertrophic cardiomyopathy in mice, Plos One, vol. 7, no. 8, p. e42805, 2012. https://doi.org/10.1371/journal.pone.0042805
[11] A. Negro, B. Brar, Y. Gu, K. Peterson, W. Vale, & K. Lee. Erbb2 is required for g protein-coupled receptor signaling in the heart, Proceedings of the National Academy of Sciences, vol. 103, no. 43, p. 15889-15893, 2006. https://doi.org/10.1073/pnas.0607499103
[12] F. Troise, M. Monti, A. Merlino, F. Cozzolino, C. Fedele, I. Krauss, et al. A novel erbb2 epitope targeted by human antitumor immunoagents, Febs Journal, vol. 278, no. 7, p. 1156-1166, 2011. https://doi.org/10.1111/j.1742-4658.2011.08041.x