Human metapneumovirus (hMPV) is a respiratory virus that was first identified in 2001. It belongs to the Paramyxoviridae family and the Pneumovirus genus, which also includes respiratory syncytial virus (RSV). Human metapneumovirus primarily infects the respiratory tract, causing respiratory infections in people of all ages, but it is particularly associated with illnesses in young children and older adults.
1. Classification of human metapneumovirus
Human metapneumovirus (hMPV) is classified within the Paramyxoviridae family, specifically under the Pneumovirus genus. The virus has a non-segmented, negative-sense single-stranded RNA genome of approximately 13.3 kilobases. It is further categorized into two main subgroups, A and B, each containing distinct genetic variants. The evolutionary origin of hMPV involves a zoonotic transmission, possibly from birds to humans. This classification aids in understanding the genetic diversity, evolution, and relationships with related respiratory viruses, such as respiratory syncytial virus (RSV). Such insights are essential for diagnostic and treatment strategies and inform ongoing efforts in vaccine development.
2. Genome and structure of human metapneumovirus
Human metapneumovirus is an enveloped, negative-sense, single-stranded RNA virus.
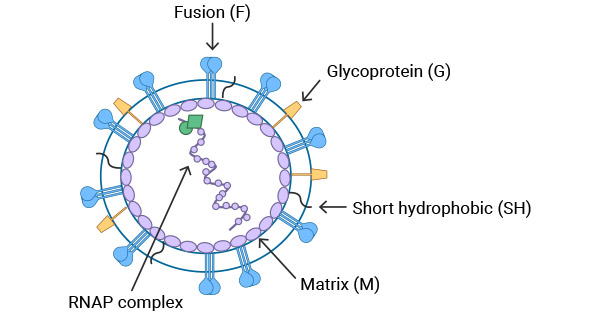
The structure of the HMPV virus is depicted in Figure 1. The matrix protein (M) is located on the inner side of the virus's lipid bilayer. The viral particle contains three types of membrane surface glycoproteins: fusion protein (F), glycoprotein (G), and short hydrophobic protein (SH). Encapsidated within the viral envelope is the ribonucleoprotein (RNAP) complex consisting of the helical, genomic RNA wrapped by the nucleoprotein (N), the viral RNA-dependent, RNA polymerase (L), phosphoprotein (P), and matrix 2 protein (M2).
Figure 1. Schematic diagram of human metapneumovirus (HMPV) virus particle [1]
3. Pathogenesis of human metapneumovirus
HMPV is transmitted through respiratory droplets in the air or by direct contact with infected individuals. The virus first infects respiratory epithelial cells. The fusion protein (F protein) and glycoprotein (G protein) on the virus surface bind to receptors on the host cell membrane, allowing the virus to enter the cell. Once inside the cell, the virus releases its genome, which is a negative-sense RNA. The RNA is transcribed into mRNA and translated into viral proteins. These proteins assist the virus in replicating its RNA genome, forming new virus particles. The newly formed virus particles then leave the infected cell, further infecting respiratory epithelial cells and spreading the virus.
To support research on the pathogenesis and prevention of HMPV, CUSABIO has responded promptly by organizing a series of research reagents related to HMPV studies.
|
Code
|
Product Name
|
Target
|
Species
|
|
CSB-EP751042HDAM
|
Recombinant Human metapneumovirus Nucleoprotein(N)
|
N
|
Human metapneumovirus(strain CAN97-83)(HMPV)
|
|
CSB-EP761526HDAM
|
Recombinant Human metapneumovirus Matrix protein(M)
|
M
|
Human metapneumovirus(strain CAN97-83)(HMPV)
|
|
CSB-EP804307HDAM
|
Recombinant Human metapneumovirus Phosphoprotein(P)
|
P
|
Human metapneumovirus (strain CAN97-83) (HMPV)
|
|
CSB-BP761526HDAM
|
Recombinant Human metapneumovirus Matrix protein(M)
|
M
|
Human metapneumovirus(strain CAN97-83)(HMPV)
|
|
CSB-YP761526HDAM
|
Recombinant Human metapneumovirus Matrix protein(M)
|
M
|
Human metapneumovirus (strain CAN97-83) (HMPV)
|
|
CSB-CF751041HDAMa2
|
Recombinant Human metapneumovirus Fusion glycoprotein F0(F)
|
F
|
Human metapneumovirus(strain CAN97-83)(HMPV)
|
|
CSB-EP751041HDAM1
|
Recombinant Human metapneumovirus Fusion glycoprotein F0(F),partial
|
F
|
Human metapneumovirus (strain CAN97-83) (HMPV)
|
4. The infection characteristics of human metapneumovirus
The infection characteristics of human metapneumovirus (hMPV) involve several key aspects that contribute to the understanding of how the virus behaves within the human host:
Respiratory Tract Tropism:
hMPV primarily infects the respiratory tract, targeting the epithelial cells lining the airways. This tropism contributes to the respiratory symptoms associated with hMPV infections.
Clinical Presentation:
Infections with hMPV can result in a spectrum of respiratory illnesses, ranging from mild upper respiratory tract infections (URIs) to more severe lower respiratory tract infections (LRIs). Common clinical manifestations include cough, nasal congestion, fever, and shortness of breath.
Severity Across Age Groups:
Although hMPV infections can affect individuals of all ages, severe cases and complications are more commonly observed in young children, the elderly, and individuals with underlying health conditions or weakened immune systems.
Seasonal Variation:
hMPV exhibits a seasonal pattern, with increased activity typically observed in late fall, winter, and early spring. This seasonality aligns with the prevalence of other respiratory viruses.
Transmission:
The virus spreads primarily through respiratory droplets generated when an infected person coughs, sneezes, or talks. Close person-to-person contact and contact with contaminated surfaces can also contribute to transmission.
Incubation Period:
The incubation period for hMPV is typically short, ranging from 2 to 5 days. This refers to the time between exposure to the virus and the onset of symptoms.
Duration of Illness:
The duration of illness caused by hMPV can vary but generally lasts for about one to two weeks. However, in severe cases or individuals with weakened immune systems, the illness may persist longer.
Coinfections:
Coinfections with other respiratory viruses or bacteria are not uncommon. hMPV may coexist with pathogens such as influenza viruses, respiratory syncytial virus (RSV), or bacteria, potentially influencing the severity of the illness.
5. Recent Research and Advances
In recent years, significant progress has been made in the research on human metapneumovirus (hMPV). A study in 2019 revealed that hMPV, along with human respirovirus 3 (HRV3), simultaneously caused outbreaks in two chimpanzee communities in Uganda, shedding new light on the virus's transmission dynamics. Furthermore, research highlighted novel antiviral activities of drugs, such as obatoclax and emetine, against hMPV and other viruses in cell cultures. A study conducted in China aimed to identify dominant meteorological factors affecting seven common respiratory viruses, including hMPV, showcasing their seasonal and regional variations.
Immunological investigations demonstrated that hMPV employs m6A modifications on its RNA to evade host immune detection, providing novel insights for potential vaccine development. These studies collectively contribute to our understanding of the biological characteristics, transmission mechanisms, and avenues for antiviral and vaccine development for hMPV.
References
[1] J V Williams, R G Cox. Breaking In: Human Metapneumovirus Fusion and Entry[J]. Viruses, 2013, 5(1).
[2] A V D Bergh, P Guillon, M V Itzstein, et al. Drug Repurposing for Therapeutic Discovery against Human Metapneumovirus Infection[J]. Antimicrobial agents and chemotherapy, 66(10).
[3] D Corti, S Bianchi, F Vanzetta, et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature 2013, 501, 439–443.
[4] J E Schuster, R G Cox, A K Hastings, et al. A Broadly Neutralizing Human Monoclonal Antibody Exhibits In Vivo Effificacy Against Both Human Metapneumovirus and Respiratory Syncytial Virus. J. Infect. Dis. 2014, 211, 216–225.
[5] H Fausther-Bovendo, H MarieEve, J Carbonneau, et al. A Candidate Therapeutic Monoclonal Antibody Inhibits Both HRSV and HMPV Replication in Mice[J]. Biomedicines, 2022, 10(10).
CUSABIO team. What Do You Need to Know Before Researching Human Metapneumovirus?. https://www.cusabio.com/c-21124.html




Comments
Leave a Comment