[1] Transferrin receptor targeting chimeras for membrane protein degradation. Nature, 2024.
[2] Candelaria, Pierre V., et al. "Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents." Frontiers in immunology 12 (2021): 607692.
[3] Jabara, Haifa H., et al. "A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency." Nature genetics 48.1 (2016): 74-78.
[4] Greene, Christopher J., et al. "Transferrin receptor 1 upregulation in primary tumor and downregulation in benign kidney is associated with progression and mortality in renal cell carcinoma patients." Oncotarget 8.63 (2017): 107052.
[5] Kawabata, Hiroshi. "Transferrin and transferrin receptors update." Free Radical Biology and Medicine 133 (2019): 46-54.
[6] Kleven, Mark D., Shall Jue, and Caroline A. Enns. "Transferrin receptors TfR1 and TfR2 bind transferrin through differing mechanisms." Biochemistry 57.9 (2018): 1552-1559.
[7] Li, Huihui, et al. "Decreasing TfR1 expression reverses anemia and hepcidin suppression in β-thalassemic mice." Blood, The Journal of the American Society of Hematology 129.11 (2017): 1514-1526.
[8] Magro, Gaetano, et al. "Aberrant expression of TfR1/CD71 in thyroid carcinomas identifies a novel potential diagnostic marker and therapeutic target." Thyroid 21.3 (2011): 267-277.
[9] Silvestri, Laura, et al. "The extrahepatic role of TFR2 in iron homeostasis." Frontiers in pharmacology 5 (2014): 93.
[10] Tang, Li-Jing, et al. "Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion." Free Radical Biology and Medicine 162 (2021): 339-352.
[11] Kawabata, Hiroshi. "Transferrin and transferrin receptors update." Free Radical Biology and Medicine 133 (2019): 46-54.
[12] Nadadur, S. S., K. Srirama, and Anuradha Mudipalli. "Iron transport & homeostasis mechanisms: their role in health & disease." Indian Journal of Medical Research 128.4 (2008): 533-544.
[13] Gammella, Elena, et al. "The transferrin receptor: the cellular iron gate." Metallomics 9.10 (2017): 1367-1375.
[14] Tsiftsoglou, Asterios S., Ioannis S. Vizirianakis, and John Strouboulis. "Erythropoiesis: model systems, molecular regulators, and developmental programs." IUBMB life 61.8 (2009): 800-830.
[15] Bayeva, Marina. Novel Regulators of Mitochondrial and Cellular Iron Homeostasis. Diss. Northwestern University, 2012.
[16] Zhao, Lingling, et al. "FLCN is a novel Rab11A-interacting protein that is involved in the Rab11A-mediated recycling transport." Journal of Cell Science 131.24 (2018): jcs218792.
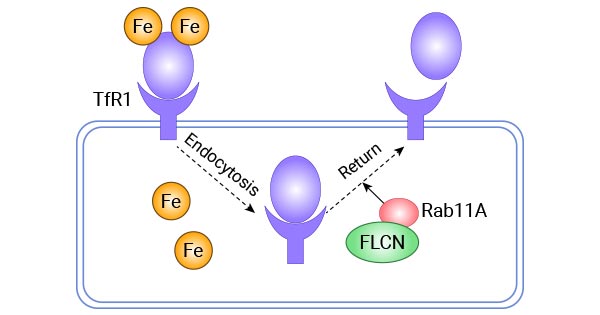
[17] Wang, Xiaojuan, et al. "FLCN regulates transferrin receptor 1 transport and iron homeostasis." Journal of Biological Chemistry 296 (2021).
[18] Ge, Xiaogang, et al. "Treatment with paraquat affects the expression of ferroptosis-related genes." Human & Experimental Toxicology 42 (2023): 09603271231167585.
[19] Wu, Hongrong, et al. "Identification and validation of transferrin receptor protein 1 for predicting prognosis and immune infiltration in lower grade glioma." Frontiers in Molecular Neuroscience 15 (2022).
[20] Wu, Hongrong, et al. "Identification and validation of transferrin receptor protein 1 for predicting prognosis and immune infiltration in lower grade glioma." Frontiers in Molecular Neuroscience 15 (2022): 972308.
[21] Klüssendorf, Malte, et al. "The Golgi-associated PDZ domain protein Gopc/PIST is required for synaptic targeting of mGluR5." Molecular Neurobiology 58.11 (2021): 5618-5634.
[22] Zhou, Jia-Huan, et al. "Ablation of TFR1 in Purkinje cells inhibits mGlu1 trafficking and impairs motor coordination, but not autistic-like behaviors." Journal of Neuroscience 37.47 (2017): 11335-11352.
[23] Warming, Hannah Kate. Haemoglobin neurotoxicity, haptoglobin scavenging and synaptic function in subarachnoid haemorrhage. Diss. University of Southampton, 2023.
[24] Parenti, Rosalba, Lucia Salvatorelli, and Gaetano Magro. "Anaplastic thyroid carcinoma: current treatments and potential new therapeutic options with emphasis on TfR1/CD71." International journal of endocrinology 2014 (2014).
[25] Ye, Jiecheng, et al. "A novel iron (II) phenanthroline complex exhibits anticancer activity against TFR1-overexpressing esophageal squamous cell carcinoma cells through ROS accumulation and DNA damage." Biochemical Pharmacology 166 (2019): 93-107.
[26] Corte-Rodriguez, Mario, et al. "Quantitative analysis of transferrin receptor 1 (TfR1) in individual breast cancer cells by means of labeled antibodies and elemental (ICP-MS) detection." Analytical chemistry 91.24 (2019): 15532-15538.
[27] Xiao, Chong, et al. "Transferrin receptor regulates malignancies and the stemness of hepatocellular carcinoma-derived cancer stem-like cells by affecting iron accumulation." PLoS One 15.12 (2020): e0243812.
[28] Cui, Can, et al. "Downregulation of TfR1 promotes progression of colorectal cancer via the JAK/STAT pathway." Cancer Management and Research 11 (2019): 6323.
[29] Liu, Qian, et al. "Significance of CD71 expression by flow cytometry in diagnosis of acute leukemia." Leukemia & lymphoma 55.4 (2014): 892-898.
[30] Jeong, Seung Min, Sunsook Hwang, and Rho Hyun Seong. "Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation." Biochemical and biophysical research communications 471.3 (2016): 373-379.
[31] Martínez;nez, Laura E., et al. "Targeting TfR1 with the ch128. 1/IgG1 Antibody Inhibits EBV-driven Lymphomagenesis in Immunosuppressed Mice Bearing EBV+ Human Primary B-cells." Molecular cancer therapeutics 20.9 (2021): 1592-1602.
[32] Chen, Chunli, et al. "Deferoxamine-induced high expression of TfR1 and DMT1 enhanced iron uptake in triple-negative breast cancer cells by activating IL-6/PI3K/AKT pathway." OncoTargets and therapy 12 (2019): 4359.
[33] Huang, Luji, et al. "Iron metabolism in colorectal cancer." Frontiers in Oncology 13 (2023).
[34] Whitnall, Megan, and Des R. Richardson. "Iron: a new target for pharmacological intervention in neurodegenerative diseases." Seminars in pediatric neurology. Vol. 13. No. 3. WB Saunders, 2006.
[35] Yu, Xiaojun, et al. "Decreased iron levels in the temporal cortex in postmortem human brains with Parkinson disease." Neurology 80.5 (2013): 492-495.
[36] Lu, Li-Na, et al. "Expression of iron transporters and pathological hallmarks of Parkinson’s and Alzheimer’s diseases in the brain of young, adult, and aged rats." Molecular neurobiology 54 (2017): 5213-5224.
[37] Cabrera, C., et al. "Relationship between iron deficiency and expression of genes involved in iron metabolism in human myocardium and skeletal muscle." International journal of cardiology 379 (2023): 82-88.
[38] Fillebeen, Carine, and Kostas Pantopoulos. "Hepatitis C virus infection causes iron deficiency in Huh7. 5.1 cells." PLoS One 8.12 (2013): e83307.
[39] Lindenbach, Brett D., and Charles M. Rice. "The ins and outs of hepatitis C virus entry and assembly." Nature Reviews Microbiology 11.10 (2013): 688-700.
[40] Bonkovsky, Herbert L., et al. "Iron and HFE or TfR1 mutations as comorbid factors for development and progression of chronic hepatitis C." Journal of Hepatology 37.6 (2002): 848-854.
[41] Kalinowska, Magdalena. Metabotropic regulation of dendritic spine structural plasticity. Diss. Yeshiva University, 2015.
[42] Zhou, Jia-Huan, et al. "Ablation of TFR1 in Purkinje cells inhibits mGlu1 trafficking and impairs motor coordination, but not autistic-like behaviors." Journal of Neuroscience 37.47 (2017): 11335-11352.
[43] Bray, Natasha. "Transferrin'bispecific antibodies across the blood–brain barrier." Nature Reviews Drug Discovery 14.1 (2015): 14-15.
[44] Pardridge, William M. "Blood–brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody." Expert opinion on drug delivery 12.2 (2015): 207-222.
[45] Shimosaki, Shunsuke, et al. "Development of a complete human IgG monoclonal antibody to transferrin receptor 1 targeted for adult T-cell leukemia/lymphoma." Biochemical and biophysical research communications 485.1 (2017): 144-151.
[46] Candelaria, Pierre V., et al. "Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents." Frontiers in immunology 12 (2021): 607692.




Comments
Leave a Comment