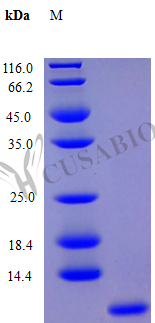
Recombinant Human Platelet Factor 4 (PF4) protein is expressed in E. coli and represents the full length of the mature protein, spanning amino acids 32 to 101. This tag-free protein is highly pure, with a purity greater than 97% as verified by SDS-PAGE. It appears to be fully biologically active, with activity confirmed through a chemotaxis bioassay using human fibroblasts at concentrations of 1.0-10 ng/ml. The endotoxin level is maintained at less than 1.0 EU/µg, determined by the LAL method.
Platelet Factor 4 (PF4) belongs to the CXC chemokine family and seems to play a significant role in modulating immune and inflammatory responses. Activated platelets release it primarily from alpha granules, and it's involved in processes such as chemotaxis, where it influences the migration of various immune cells. Researchers also study PF4 for its involvement in coagulation and its potential impact on angiogenesis, making it a protein of interest across multiple research fields.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Chemotaxis Assay Development and Optimization
This recombinant PF4 protein is confirmed to be biologically active in chemotaxis assays (active at 1.0-10 ng/ml using human fibroblasts) and suitable as a positive control and reference standard. The defined concentration range provides reliable dosing parameters for experimental design. High purity (>97%) and low endotoxin levels ensure minimal interference in cell-based assays. However, researchers should validate the optimal concentration range for specific cell types beyond fibroblasts, as chemotactic responses may vary.
2. Antibody Development and Validation
The tag-free, high-purity PF4 is appropriate for antibody development, and the confirmed biological activity suggests proper folding for conformational epitope presentation. However, since the protein is expressed in E. coli and lacks mammalian post-translational modifications, antibodies generated should be validated against native PF4 from human platelets to ensure recognition of physiologically relevant forms in clinical samples.
3. Protein-Protein Interaction Studies
The biologically active PF4 can be used to study interactions with known partners (heparin, glycosaminoglycans, receptors), but researchers should note that the E. coli expression lacks natural glycosylation, which may affect some binding interactions. The confirmed chemotactic activity supports proper folding for core interactions, but findings should be validated with native PF4 when studying modification-sensitive binding.
4. Cell Migration and Wound Healing Research
The demonstrated chemotactic activity on fibroblasts makes this PF4 suitable for migration and wound healing studies. However, the optimal concentrations may vary across different assay formats (scratch assays vs. transwell systems). Researchers should perform dose-response optimization for their specific experimental setup rather than relying solely on the fibroblast chemotaxis data.
5. Comparative Functional Analysis
This protein serves as an excellent reference standard for comparing PF4 variants or expression systems. The established fibroblast chemotaxis assay provides a standardized platform for structure-function studies. However, researchers should confirm that any observed functional differences are due to structural changes rather than batch-to-batch variability when comparing with other PF4 preparations.
Final Recommendation & Action Plan
This E. coli-expressed human PF4 is a well-characterized reagent suitable for all proposed applications, with its biological activity confirmed in fibroblast chemotaxis assays. For immediate use, employ it within the validated 1.0-10 ng/ml range for chemotaxis and migration studies, but perform optimization for specific cell types and assay formats. For antibody development, this protein is ideal for immunization, but validate resulting antibodies against native platelet-derived PF4 for clinical relevance. While the E. coli expression produces a non-glycosylated form, the demonstrated bioactivity confirms proper folding for core PF4 functions. For interaction studies involving glycosaminoglycan binding, the recombinant protein should perform comparably to native PF4 since the primary heparin-binding domain is contained within the mature sequence. Always include appropriate controls and consider that, while highly pure and active, this preparation represents one specific form of PF4 that may not capture all nuances of endogenous platelet-released protein complexes.