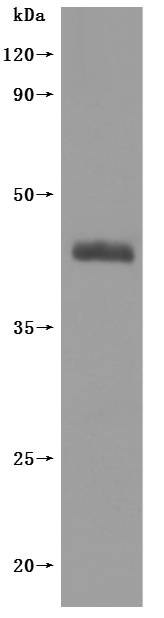
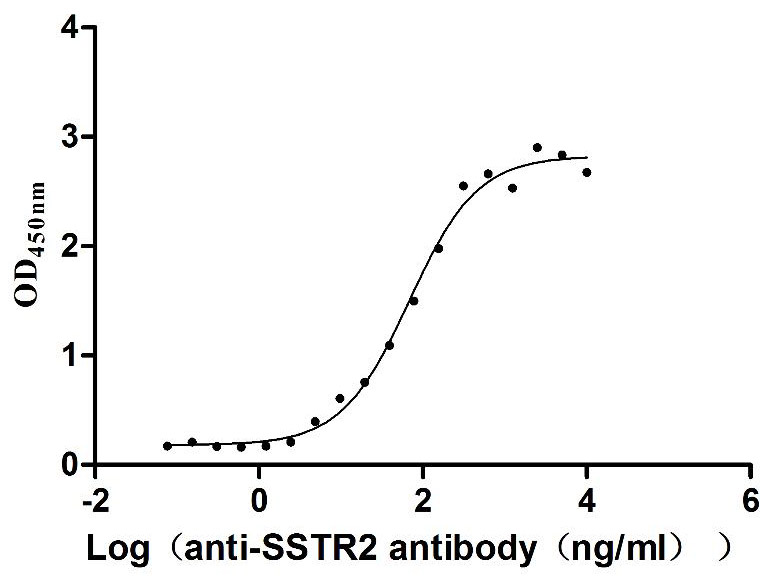
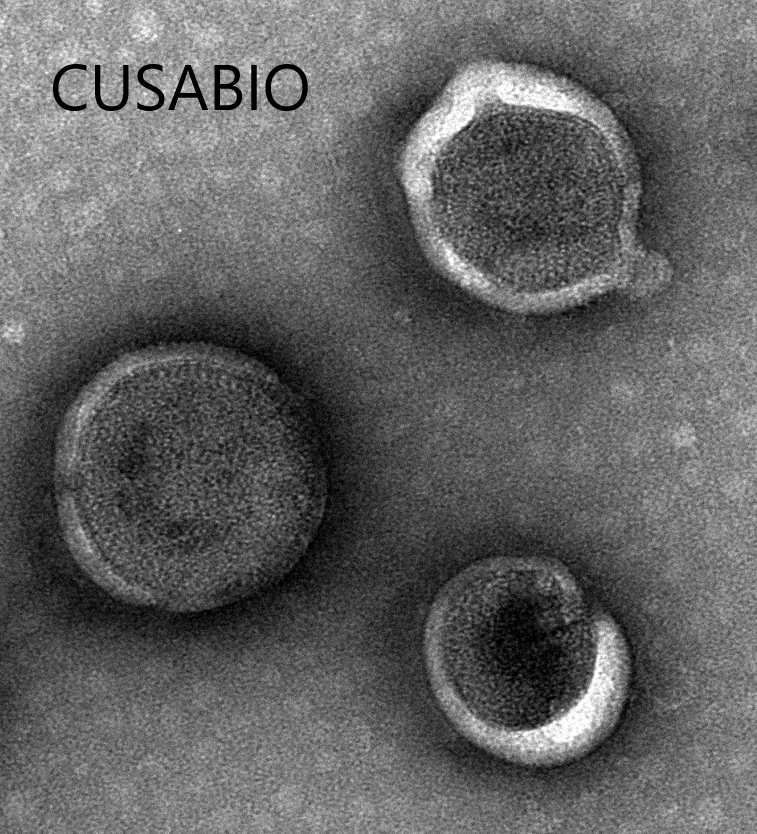
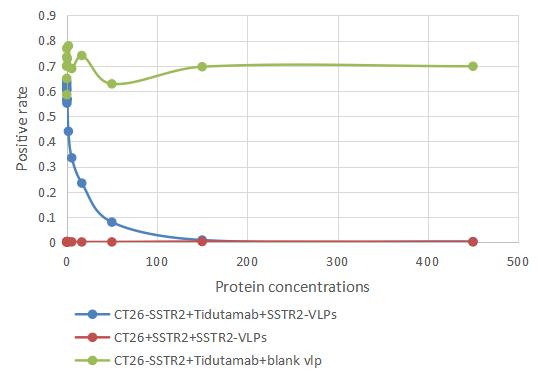
The recombinant human somatostatin receptor type 2 (SSTR2)-VLPs are produced using virus-like particles (VLPs) technology. Clone the target gene including the human SSTR2 encoding gene with the C-terminal 6xHis-tag gene into an expression vector along with the viral capsid proteins. Introduce the expression vector into the mammalian cells. Culture the cells, induce protein expression, and harvest the cells to obtain the VLPs. Purify the VLPs using affinity chromatography. CUSABIO has characterized the VLPs with TEM and validated the functionality of the recombinant SSTR2 protein through ELISA. The endotoxin of this protein is less than 1.0 EU/ug as determined by the LAL method.
SSTR2 is a protein that's pretty important in the body's workings. It's found in various tissues and helps regulate the release of growth hormone (GH), which is tied to conditions like acromegaly [1]. SSTR2 works by picking up signals from outside the cell and passing them inside using different pathways [2]. It also has a role in controlling the release of interferon‐γ in certain immune cells [3]. Besides, SSTR2 seems to have a say in how cells in our gums react to things like inflammation, infections, and even obesity-related signals [4][5]. Plus, it helps keep luteinizing hormone (LH) levels steady during breastfeeding [6]. When it comes to acromegaly, SSTR2 steps in to keep GH levels in check and shrink tumors [1]. It's found in the gut's lining and nerve cells [7].
Sometimes SSTR2's job can be thrown off by changes in how our genes are marked, like methylation, which can make it less effective [8][9]. There's also evidence linking SSTR2 to nerve damage after a stroke, where it seems to get more active in certain brain areas [10]. Plus, it's been put into the class B receptor, which helps us understand how it works with other proteins in the cell [11]. Understanding this classification helps us grasp how SSTR2 interacts with different partners inside the cell.
References:
[1] S. Melmed, Acromegaly pathogenesis and treatment, Journal of Clinical Investigation, vol. 119, no. 11, p. 3189-3202, 2009. https://doi.org/10.1172/jci39375
[2] J. Zhao, H. Fu, J. Yu, W. Hong, X. Tian, J. Qiet al., Prospect of acromegaly therapy: molecular mechanism of clinical drugs octreotide and paltusotine, Nature Communications, vol. 14, no. 1, 2023. https://doi.org/10.1038/s41467-023-36673-z
[3] M. Ho, L. Yung, J. Chan, J. Chan, C. Wong, & Y. Wong, Gα14 links a variety of gi‐ and gs‐coupled receptors to the stimulation of phospholipase c, British Journal of Pharmacology, vol. 132, no. 7, p. 1431-1440, 2001. https://doi.org/10.1038/sj.bjp.0703933
[4] S. Nagarajan, S. Babu, S. Kulkarni, A. Vadivelu, P. Devaraju, H. Sohnet al., Understanding the influence of lipid bilayers and ligand molecules in determining the conformational dynamics of somatostatin receptor 2, Scientific Reports, vol. 11, no. 1, 2021. https://doi.org/10.1038/s41598-021-87422-5
[5] S. Memmert, A. Damanaki, M. Nokhbehsaim, A. Nogueira, S. Eick, J. Cirelliet al., Regulation of somatostatin receptor 2 by proinflammatory, microbial and obesity-related signals in periodontal cells and tissues, Head & Face Medicine, vol. 15, no. 1, 2019. https://doi.org/10.1186/s13005-018-0185-1
[6] A. Sugimoto, H. Tsuchida, M. Nagae, N. Inoue, Y. Uenoyama, & H. Tsukamura, Central somatostatin-somatostatin receptor 2 signaling mediates lactational suppression of luteinizing hormone release via the inhibition of glutamatergic interneurons during late lactation in rats, Journal of Reproduction and Development, vol. 68, no. 3, p. 190-197, 2022. https://doi.org/10.1262/jrd.2022-009
[7] J. Allen, A. Canty, S. Schulz, P. Humphrey, P. Emson, & H. Young, Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of sstr2 knockout/lacz knockin mice, The Journal of Comparative Neurology, vol. 454, no. 3, p. 329-340, 2002. https://doi.org/10.1002/cne.10466
[8] A. Wang, Y. Yuan, H. Chu, Y. Gao, J. Zheng, Q. Jiaet al., Somatostatin receptor 2: a potential predictive biomarker for immune checkpoint inhibitor treatment, Pathology & Oncology Research, vol. 28, 2022. https://doi.org/10.3389/pore.2022.1610196
[9] H. Kim, J. Park, B. Yoon, K. Haam, H. Heo, J. Kimet al., Aberrant methylation of somatostatin receptor 2 gene is initiated in aged gastric mucosa infected with helicobacter pylori and consequential gene silencing is associated with establishment of inflammatory microenvironment in vitro study, Cancers, vol. 14, no. 24, p. 6183, 2022. https://doi.org/10.3390/cancers14246183
[10] R. Stumm, C. Zhou, S. Schulz, M. Endres, G. Kronenberg, J. Allenet al., Somatostatin receptor 2 is activated in cortical neurons and contributes to neurodegeneration after focal ischemia, Journal of Neuroscience, vol. 24, no. 50, p. 11404-11415, 2004. https://doi.org/10.1523/jneurosci.3834-04.2004
[11] F. Gatto, N. Biermasz, R. Feelders, J. Kros, F. Dogan, A. Lelyet al., Low beta-arrestin expression correlates with the responsiveness to long-term somatostatin analog treatment in acromegaly, Acta Endocrinologica, vol. 174, no. 5, p. 651-662, 2016. https://doi.org/10.1530/eje-15-0391