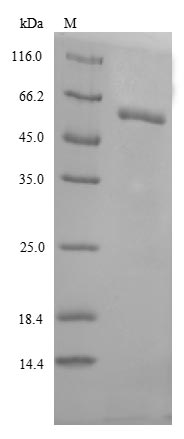
Recombinant Human Lysosomal acid glucosylceramidase (GBA) is produced in E. coli and contains the complete mature protein sequence spanning amino acids 40 to 536. The product comes without any tags, which appears to reduce interference in downstream applications. SDS-PAGE analysis confirms purity levels above 85%. This recombinant protein is intended for research use only and may offer reliability and consistency in experimental assays.
Lysosomal acid glucosylceramidase (GBA) serves as a critical enzyme that breaks down glucocerebrosides into glucose and ceramide within the lysosome. Its activity seems essential for proper cellular lipid metabolism and represents a key component in the lysosomal degradation pathway. Scientists show significant interest in GBA due to its role in various biological processes and its potential implications in metabolic studies.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant Human GBA1 is produced in an E. coli expression system. GBA1 is a complex eukaryotic enzyme that requires precise folding, glycosylation, and lysosomal targeting for full bioactivity. The E. coli system lacks glycosylation machinery and eukaryotic chaperones, making it unlikely to produce a correctly folded and bioactive form of this multi-domain protein requiring post-translational modifications. Although the protein is full-length mature (40-536aa) and tag-free - favorable for native conformation - the E. coli expression system significantly increases the risk of misfolding. Since activity is unverified, the protein cannot be assumed to be correctly folded or bioactive without experimental validation (e.g., enzymatic activity assays).
1. Antibody Development and Validation
This recombinant GBA1 protein could serve as an immunogen for generating polyclonal or monoclonal antibodies against human lysosomal acid glucosylceramidase. The absence of tags suggests resulting antibodies will likely recognize native epitopes without artificial sequence interference. The >85% purity should be adequate for immunization protocols. However, if misfolded, antibodies may recognize linear but not conformational epitopes of native GBA1. Validation against native GBA1 from mammalian sources is recommended.
2. Protein-Protein Interaction Studies
The tag-free, full-length GBA1 could theoretically be used in pull-down assays to identify binding partners. However, if misfolded, interactions with true biological partners (e.g., activator protein Saposin C) may be non-physiological, leading to false positives/negatives. This application should only be pursued after confirming proper folding and activity. Results without validation could be misleading.
3. Biochemical Characterization and Enzyme Kinetics
This protein is suitable for biochemical analysis, like stability studies and pH optimization. However, kinetic studies (Km, Vmax) require a functional enzyme. The tag-free nature is advantageous, but E. coli-expressed GBA1 likely requires refolding optimization. Initial validation of folding state (e.g., via circular dichroism) and enzymatic activity is essential before reliable kinetic analysis.
4. Substrate Binding and Inhibitor Screening Assays
The protein could be used for binding studies with substrates or inhibitors using techniques like surface plasmon resonance. However, if misfolded, the active site conformation may be altered, leading to binding data that does not reflect true biological activity. This application is high-risk without prior functional validation.
Final Recommendation & Action Plan
Given the high probability of misfolding in E. coli for this complex eukaryotic enzyme, it is crucial to first validate the protein's conformation and function. Recommend performing: 1) Biophysical characterization (e.g., size-exclusion chromatography for oligomeric state, circular dichroism for secondary structure) to assess folding; 2) Functional enzymatic activity assays using synthetic substrates (e.g., 4-MUG) to confirm bioactivity. If validated, the protein can be used for all described applications; if not, focus on antibody development and avoid functional studies. The tag-free design is beneficial, but confirmation of folding quality is essential. For reliable functional studies, consider obtaining GBA1 from a mammalian expression system capable of proper glycosylation and folding.