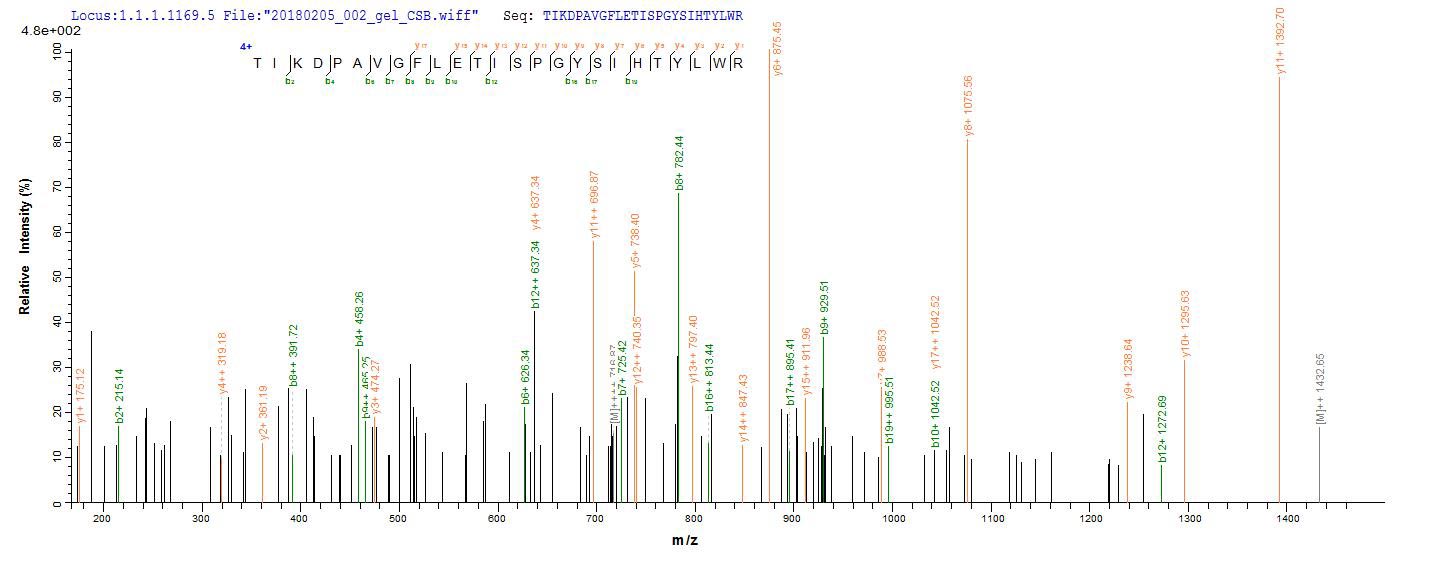
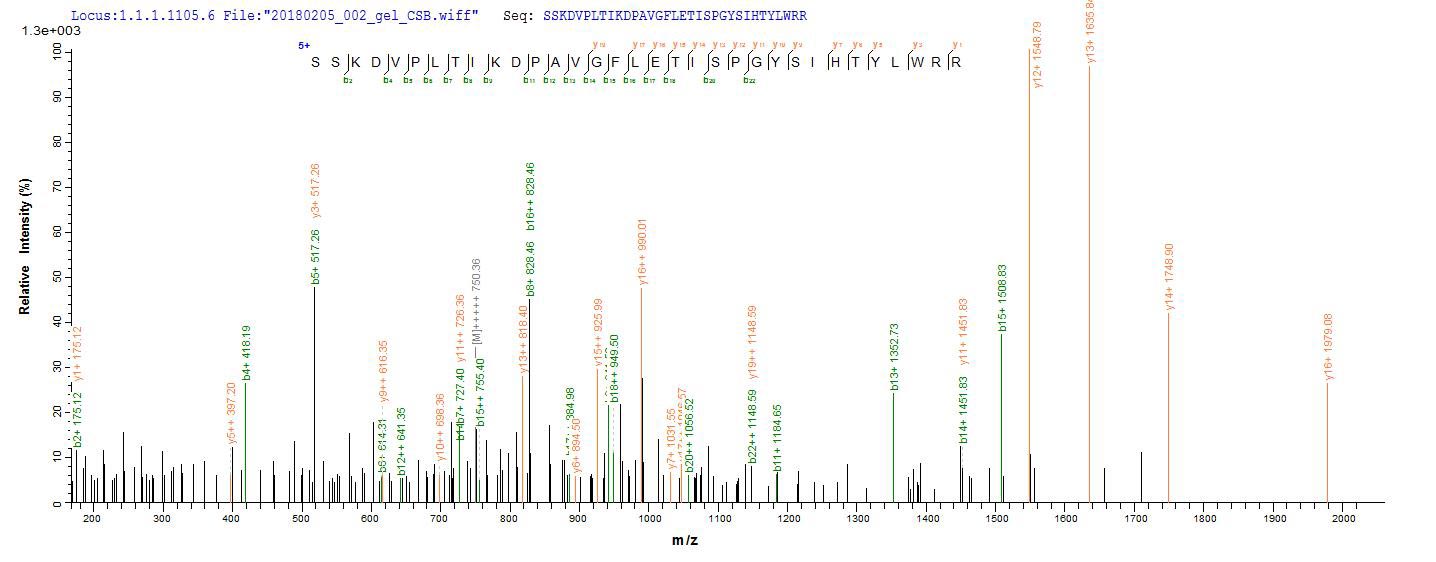
To synthesize recombinant human lysosomal acid glucosylceramidase (GBA1), the target gene encoding the 40-536aa of human GBA1 is first isolated, with an N-terminal 10xHis-SUMO-tag and C-terminal Myc-tag. This gene is cloned into an expression vector, which is introduced into E. coli cells via transformation. The E. coli cells express the recombinant GBA1 protein, which is subsequently harvested from the cell lysate. The protein is purified using affinity chromatography. Finally, the protein's purity is determined by SDS-PAGE, reaching up to 85%.
The human GBA1 gene encodes the lysosomal enzyme beta-glucocerebrosidase (GCase), which plays a crucial role in glycosphingolipid substrate metabolism [1]. Mutations in GBA1 lead to GCase deficiency, resulting in the accumulation of glucosylceramide and subsequent lysosomal dysfunction [2]. These mutations are associated with Gaucher disease, a lysosomal storage disorder characterized by GlcCer accumulation [2]. GBA1 mutations are also linked to an increased risk for synucleinopathies such as Parkinson's disease and dementia with Lewy bodies [3][4]. GBA1 deficiency can accelerate the progression of pathology in models of synucleinopathies [5]. Furthermore, GBA1 mutations negatively affect physiological α-synuclein tetramers, leading to the destabilization of multimers and accumulation of monomers [6].
References:
[1] N. Polinski, T. Martinez, A. Gorodinsky, R. Gareus, M. Sasner, M. Herberthet al., Decreased glucocerebrosidase activity and substrate accumulation of glycosphingolipids in a novel gba1 d409v knock-in mouse model, Plos One, vol. 16, no. 6, p. e0252325, 2021. https://doi.org/10.1371/journal.pone.0252325
[2] E. Schejter, S. Bialik, A. Shkedy, V. Levin-Salomon, S. Levin-Zaidman, & A. Kimchi, Death by over-eating: the gaucher disease associated gene gba1, identified in a screen for mediators of autophagic cell death, is necessary for developmental cell death in drosophila midgut, Cell Cycle, vol. 16, no. 21, p. 2003-2010, 2017. https://doi.org/10.1080/15384101.2017.1380134
[3] S. Sardi, P. Singh, S. Cheng, L. Shihabuddin, & M. Schlossmacher, Mutant <i>gba1</i> expression and synucleinopathy risk: first insights from cellular and mouse models, Neurodegenerative Diseases, vol. 10, no. 1-4, p. 195-202, 2012. https://doi.org/10.1159/000335038
[4] S. Sardi, J. Clarke, C. Viel, M. Chan, T. Tamsett, C. Treleavenet al., Augmenting cns glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other gaucher-related synucleinopathies, Proceedings of the National Academy of Sciences, vol. 110, no. 9, p. 3537-3542, 2013. https://doi.org/10.1073/pnas.1220464110
[5] D. Kim, H. Hwang, S. Choi, S. Kwon, S. Lee, J. Parket al., D409h gba1 mutation accelerates the progression of pathology in a53t α-synuclein transgenic mouse model, Acta Neuropathologica Communications, vol. 6, no. 1, 2018. https://doi.org/10.1186/s40478-018-0538-9
[6] S. Kim, S. Yun, S. Lee, G. Umanah, V. Bandaru, X. Yinet al., Gba1 deficiency negatively affects physiological α-synuclein tetramers and related multimers, Proceedings of the National Academy of Sciences, vol. 115, no. 4, p. 798-803, 2018. https://doi.org/10.1073/pnas.1700465115