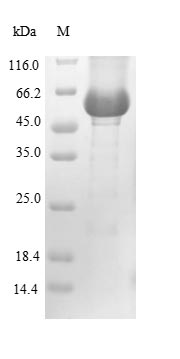
Recombinant human lysosomal acid glucosylceramidase (GBA1), expressed from E. coli, is a high-purity protein with over 85% purity, validated by SDS-PAGE. This protein contains the full length mature human GBA1 (40-536aa) and is labeled with an N-terminal 10xHis-tag for enhanced purification and functionality in experiments. Available as a liquid or lyophilized powder, this recombinant GBA1 is ideal for studies on neuroscience, providing reliable performance in experimental settings.
Human GCase, encoded by the GBA1 gene, is a critical enzyme involved in the metabolism of glycosphingolipids, particularly glucosylceramide (GlcCer). This enzyme catalyzes the hydrolysis of GlcCer into glucose and ceramide, thereby playing a vital role in lipid degradation within lysosomes [1][2]. Mutations in the GBA1 gene are responsible for Gaucher disease, the most prevalent lysosomal storage disorder [3][4]. The dysfunction of GCase leads to the accumulation of glucosylceramide in lysosomes, particularly in macrophages, which can result in various clinical manifestations including splenomegaly, hepatomegaly, and skeletal abnormalities [5][6].
References:
[1] M. Karataş, S. Dogan, E. Spahiu, A. Ašić, L. Bešić, & Y. Turan, Enzyme kinetics and inhibition parameters of human leukocyte glucosylceramidase,, 2020. https://doi.org/10.1101/2020.02.23.961599
[2] G. Grabowski, G. Andria, A. Baldellou, P. Campbell, J. Charrow, I. Cohenet al., Pediatric non-neuronopathic gaucher disease: presentation, diagnosis and assessment. consensus statements, European Journal of Pediatrics, vol. 163, no. 2, p. 58-66, 2004. https://doi.org/10.1007/s00431-003-1362-0
[3] J. Sheth, R. Bhavsar, M. Mistri, D. Pancholi, A. Bavdekar, A. Dalalet al., Gaucher disease: single gene molecular characterization of one-hundred indian patients reveals novel variants and the most prevalent mutation, BMC Medical Genetics, vol. 20, no. 1, 2019. https://doi.org/10.1186/s12881-019-0759-1
[4] M. Offman, M. Król, I. Silman, J. Sussman, & A. Futerman, Molecular basis of reduced glucosylceramidase activity in the most common gaucher disease mutant, n370s, Journal of Biological Chemistry, vol. 285, no. 53, p. 42105-42114, 2010. https://doi.org/10.1074/jbc.m110.172098
[5] L. Sousa, M. Rocha, R. Mendes, & R. Júnior, Oral health of a child being treated for subtype i gaucher's disease, Special Care in Dentistry, vol. 34, no. 2, p. 100-104, 2013. https://doi.org/10.1111/scd.12026
[6] M. Dahl, A. Doyle, K. Olsson, J. Månsson, A. Marques, M. Mirzaianet al., Lentiviral gene therapy using cellular promoters cures type 1 gaucher disease in mice, Molecular Therapy, vol. 23, no. 5, p. 835-844, 2015. https://doi.org/10.1038/mt.2015.16