On October 27, 2020, Akeso, Inc. announced the completion of its first clinical trial in New Zealand for its innovative drug candidate CD73 monoclonal antibody named AK119, which was administered to healthy subjects. AK119 is a humanized monoclonal antibody drug, which can significantly inhibit the enzymatic activity of CD73. AK119 can effectively activate B cell and enhance antibody production. AK119 is intended to be used to treat patients with SARS-CoV-2 (COIVD-19). Currently, the U.S. Food and Drug Administration (FDA) has approved the clinical trial application of AK119 for the treatment of patients with mild COVID-19. In fact, CD73 antibody is often used in anti-tumor therapy. Today, let’s talk about CD73 and tumors.
1. What is The Structure of CD73?
CD73, also known as Ecto-5'-Nucleotidase, a cell surface enzyme encoded by the NT5E gene, is a major enzyme catalyzing the formation of extracellular adenosine from AMP [1]. The gene for human CD73 is located on chromosome 6q14-921. CD73 is conservative in different species. For example, the cDNA sequence of human CD73 has 86% homology with that of mouse [2].
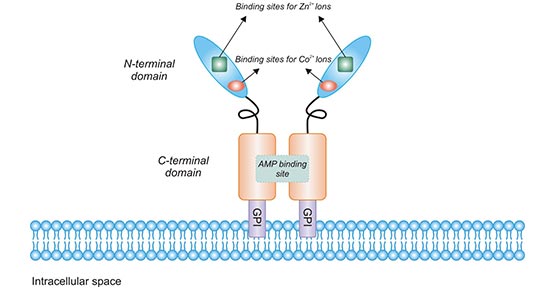
CD73 consists of two identical 70 kDa subunits tethered by non-covalent bonds (Figure 1). After the 1-26aa residues at the N-terminal and 550-574aa at the C-terminal of the CD73 molecule are removed, the 27-549aa is the region of mature CD73. In the form of mature CD73, the 26-amino acid signal peptide in the N-terminus coordinates the binding of two catalytic divalent metal ions (Zn2+ and Co2+). The C-terminus provides a binding site for AMP [3]. A portion of approximatively 25 hydrophobic amino acid residues at the C-terminus is replaced by a GPI anchor, which mediates binding of the mature protein to the cell membrane via the C-terminal Ser-523 residue. This Ser-523 residue is linked to complex oligosaccharides and sphingolipid sphingoid groups. Note that CD73 does not have protein fragments embedded in the cell membrane.
Figure 1. The structure of CD73
*This figure is derived from the publication on Trends in Cancer [4]
Furthermore, in addition to the form of CD73 anchored to the cell membrane, there is also a truncated soluble form. Soluble CD73 retains ecto-5' nucleotidase activity [5]. This soluble form can be shed from the membrane by phosphatidylinositol-specific phospholipase hydrolyzing GPI ankyrin or proteolytic enzymes and circulate freely in blood and other biological fluids [6]. Soluble nucleotidase may represent an important auxiliary effect system for the local inactivation of acutely elevated nucleotides, especially at sites of injury and inflammation [4].
2. Where is The CD73 Distributed?
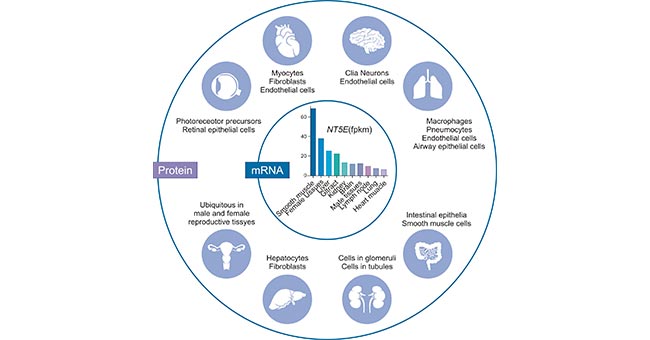
CD73 is widely expressed on the surface of human tissue cells, including liver cells, fibroblasts, endothelial cells, lymphocytes and glial cells, and proximal tubular epithelial cells. As the figure 2 shows, outer circle depicts the various tissues and cell types where CD73 protein has been detected by immune-histochemical (IHC) methods, as reported by the Human Protein Atlas project. Note that expression in the eye is based on literature reports. Inner circle depicts human NT5E gene expression (in order of abundance) based on Human Protein Atlas data.
Figure 2. Tissue-specific expression of human CD73
*This figure is derived from the publication on Am J Physiol Cell Physiol [1]
In addition, the expression level of CD73 in B cells from human cord blood is low, and its expression increases to adult levels when babies reach 6 months of age. CD73 is expressed in about 75% of adult B lymphocytes in peripheral blood, and its expression also increases during the maturation of human T lymphocytes. On thymocytes, the enzymatic activity of CD73 is almost undetectable, but it can be easily detected on some T lymphocytes in peripheral blood, spleen or lymph nodes.
3. What is the function of CD73?
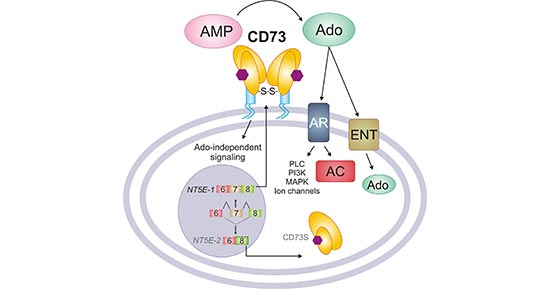
CD73 is a metabolic immune checkpoint that coordinates the critical homeostasis of extracellular adenosine levels. It undergoes homo-dimerization mediated by N-linked glycosylation and disulfide bonds, and is anchored to the cell membrane surface through GPI. It is one of the rate-limiting enzymes in the extracellular purine metabolism pathway [7]. It exists on the surface of many cell types, including endothelial cells, lymphocytes, stromal cells and tumor cells. Its enzymatic activity is closely related to extracellular phosphatase (CD39). CD39 can produce AMP, which is the substrate of CD73 [8]. The extracellular adenosine produced by CD73 can interact with adenosine receptors (A1R, A2AR, A2BR, and A3R) to activate downstream G protein-coupled signals and regulate adenylate cyclase (AC) activity, or by balancing the nuclear Glycoside transporters (ENTs) transport it into the cell.
As shown in Figure 3, adenosine can also regulate phospholipase C (activated by A1R, A2BR and A3R), MAP kinase (activated by all AR subtypes), PI3K (activated by A1R and A3R), potassium (KATP) and calcium Channel (activated by A1R). Among the many physiological responses regulated by adenosine receptors, epithelial ions and fluid transport, tissue barrier maintenance, hypoxia, ischemic preconditioning, and inflammation have been shown to be closely related to CD73 activity [9].
Figure 3. The function of CD73
*This figure is derived from the publication on Am J Physiol Cell Physiol [1]
4. CD73 and Tumor
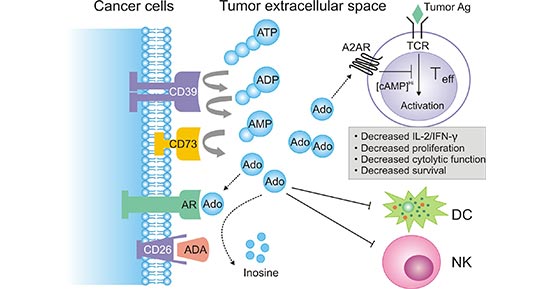
The metabolism of tumor microenvironment (TME) is active and rich in adenosine. Adenosine is an effective immunosuppressive molecule that can strongly inhibit various immune cells, especially T cell proliferation, cytotoxicity and cytokine production [10]. The primary pathway of extracellular adenosine generation in the TME is the sequential action of CD39 and CD73, both of which can be induced by tumor hypoxia, to degrade ATP (Figure 4). It is very likely that coordinated expression and activity of CD39/CD73 (increased) and ecto-adenosine deaminase ADA (decreased) which in human cells can be bound to the cell surface by CD26 contribute to the elevated levels of extracellular adenosine during tumor progression. High concentrations of adenosine suppress antitumor T cell activation, survival and effector function via A2AR engagement. Meanwhile, adenosine may trigger certain adenosine receptors (AR) on cancer cells to promote tumor cell chemotaxis and metastasis. In addition, adenosine can negatively modulate differentiation and function of dendritic cells (DCs), and further inhibit natural killer (NK) cell proliferation and cytolytic function [11] [12].
Figure 4. a diagram of extracellular adenosine metabolism in tumor-induced immune suppression
*This figure is derived from the publication on Cancer Res [12]
Accumulating studies have confirmed that tumor cells can also express CD73 and release adenosine, and its expression and activity are closely related to tumor invasion and metastasis [13] [14]. Moreover, in tumor cells, the extracellular adenosine produced by CD73 is sufficient to mediate immune escape and promote tumor growth and metastasis. In addition to the immune regulation of CD73 by tumor cells, CD73 also affects many aspects of tumorigenesis, such as proliferation, adhesion, angiogenesis and metastasis. It promotes tumor cell proliferation by regulating cell cycle, apoptosis and signaling pathways such as EGFR, β-catenin / cyclin D1, VEGF and AKT / ERK. Without relying on its enzymatic function, CD73 can also promote cell adhesion, migration and cancer cell invasion.
CD73-adenosine mediated immunosuppression can control the inflammatory response in the microenvironment of tissues that are stressed or damaged. The lack of CD73 expression may imply physiological wound healing and immune regulation in the tissue microenvironment. However, within the TME, metabolic pressure accumulates as the tumor progresses, which leads to dysregulation of CD73 expression and activity in cancers including breast cancer, metastatic melanoma and ovarian cancer [15]. Overexpression of CD73 in tumors not only leads to metastasis and anthracycline resistance, but also leads to immune escape due to imbalance of adenosine production.
5. The Clinical Significance of CD73 Research
CD73 is an emerging tumor immune target, which is closely related to the occurrence, development, metastasis, and poor prognosis of tumors. Preclinical studies have also fully proved that CD73 can promote the immune escape of tumors. Drugs targeting CD73 can curb tumor development and metastasis, and can be combined with PD-L1 monoclonal antibodies and/or A2AR inhibitors. The combination of anti-tumor therapies targeting CD73 and other anti-tumor therapies has received extensive attention in anti-tumor treatments to produce synergistic effects. However, targeted CD73 therapy can also cause damage to normal tissues. Studies have found that inhibiting mouse CD73 gene expression can cause fluid leakage in multiple organs and vessels. Therefore, anti-tumor treatments targeting CD73 with high specificity need to be further studied. Here, we summarized the latest CD73 target clinical drug information.
| Drug Name |
Phase |
Company |
Disease |
| CPI-006 |
Phase I |
Corvus |
Triple negative breast cancer, non-small cell lung cancer, renal cell carcinoma, endometrial cancer, head and neck squamous cell carcinoma, colorectal cancer, metastatic castration-resistant prostate cancer, sarcoma, bladder cancer, ovarian cancer, cervical cancer, Pancreatic cancer |
| Anti-CD73 monoclonal antibody (Bristol-Myers Squibb) |
Phase I |
Bristol Myers Squibb |
Cancer |
| TJ-004309 |
Phase II |
IMAB, Tracon Pharma |
Solid tumor, metastatic cancer |
| LY-3475070 |
Phase I |
Eli Lilly |
Cancer |
| BMS-986179 |
Phase II |
Bristol Myers Squibb |
Solid tumor |
| Oleclumab |
Phase II |
MedImmune |
Non-small cell lung cancer, prostate cancer, breast cancer, ovarian cancer, pancreatic cancer |
| AB-680 |
Phase II |
Arcus Biosciences |
Castration resistant prostate cancer |
| SRF-373 |
Phase I |
Surface Oncology, Novartis |
Solid tumor |
CD73 protein
● Recombinant Human 5'-nucleotidase(NT5E) (Active) (Code: CSB-MP723415HU)
High Purity Validated by SDS-PAGE

(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
Excellent Bioactivity Validated by Functional ELISA
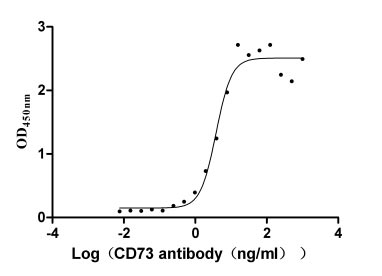
Immobilized CD73 at 2 μg/ml can bind Anti- CD73 Rabbit Monoclonal Antibody(CSB-RA978310A0HU), the EC50 is 3.212-4.525 ng/ml.
References
[1] Marquet Minor, Karel P. Alcedo, Rachel A. Battaglia, et al. Cell type- and tissue-specific functions of ecto-5´-nucleotidase (CD73) [J]. Am J Physiol Cell Physiol. 2019.
[2] Pouya Joolharzadeh, Cynthia St Hilaire. CD73 (Cluster of Differentiation 73) and the Differences Between Mice and Human [J]. Arterioscler. Thromb. Vasc. Biol. 2019.
[3] Strater, N. Ecto-50-nucleotidase: Structure function relationships [J]. Purinergic Signal. 2006, 2, 343–350.
[4] Luca Antonioli, Gennady G. Yegutkin, Pál Pacher, et al. Anti-CD73 in Cancer Immunotherapy: Awakening New Opportunities [J]. Trends in Cancer. 2016, 2(2):95-109.
[5] Antonioli, L. et al. CD39 and CD73 in immunity and inflammation [J]. Trends Mol. Med. 2013, 19, 355–367.
[6] Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade [J]. Biochim. Biophys. Acta. 2008, 1783, 673–694.
[7] Alcedo KP, Guerrero A, Basrur V, et al. Tumor‐selective altered glycosylation and functional attenuation of CD73 in human hepatocellular carcinoma [J]. Hepatology Communications In Press. 2019.
[8] Antonioli L, Pacher P, Vizi ES, et al. CD39 and CD73 in immunity and inflammation [J]. Trends Mol Med. 2013, 19:355-367.
[9] Borea PA, Gessi S, Merighi S, et al. Pharmacology of Adenosine Receptors: The State of the Art [J]. Physiol Rev. 2018, 98: 1591‐1625.
[10] Ochoa de Olza M, Navarro Rodrigo B, Zimmermann S, et al. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development [J]. The Lancet Oncology. 2020, 21(9):e419-e30.
[11] Vijayan D, Young A, Teng MWL, et al. Targeting immunosuppressive adenosine in cancer [J]. Nature reviews Cancer. 2017, 17(12):709-24.
[12] Bin Zhang. CD73: A novel target for cancer immunotherapy [J]. Cancer Res. 2010, 70(16): 6407–6411.
[13] Chen S, Wainwright DA, Wu JD, et al. Cd73: an emerging checkpoint for cancer immunotherapy [J]. Immunotherapy. 2019, 11:983-997.
[14] Meejeon Roh, Derek A Wainwright, Jennifer D Wu, et al. Targeting CD73 to augment cancer immunotherapy [J]. Current Opinion in Pharmacology. 2020, 53:1–11.
[15] Bertrand Allard, Maria Serena Longhi, Simon C. Robson, et al. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets [J]. Immunological Reviews. 2017, 276: 121–144.
CUSABIO team. CD73, What a Powerful Therapy Target in Tumor Immune. https://www.cusabio.com/c-20993.html






Comments
Leave a Comment